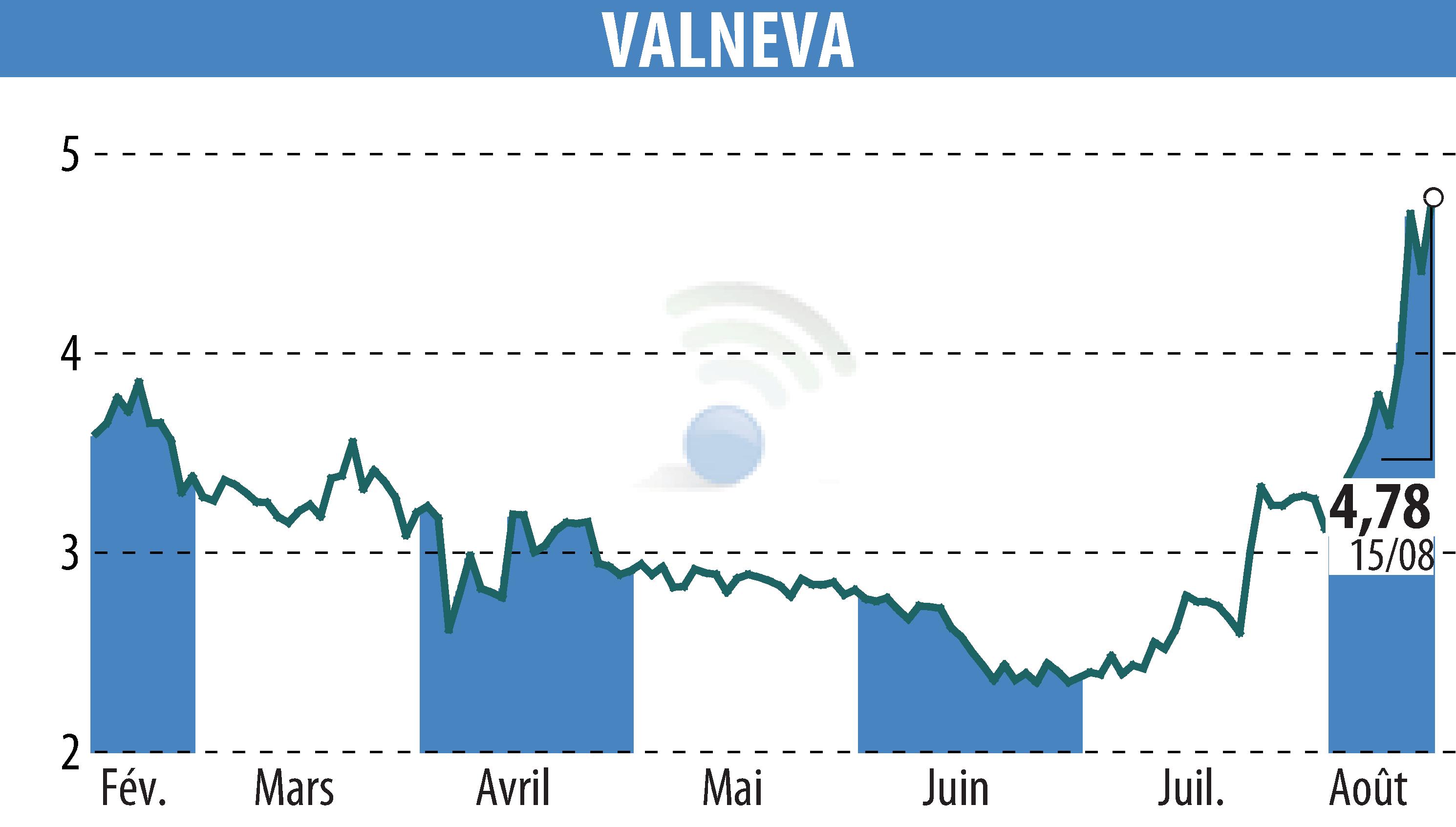
sur VALNEVA (EPA:VLA)
Valneva's IXCHIQ® Vaccine Authorized in Canada for Ages 12 and Older
Valneva SE announced Health Canada's authorization of its chikungunya vaccine, IXCHIQ®, for individuals 12 years of age and older. This decision expands the initial authorization granted to adults, similar to the extension obtained in Europe for adolescents. Data submitted to Health Canada demonstrate prolonged antibody persistence over 24 months in 97% of participants.
Dr. Juan Carlos Jaramillo emphasized the importance of covering all age groups, as chikungunya spreads to previously unaffected areas. Through a partnership with CEPI, Valneva hopes to expand access to its vaccine in low- and middle-income countries.
As a reminder, IXCHIQ® was the first chikungunya vaccine approved in Brazil. This approval in Canada could facilitate other global approvals.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de VALNEVA
