sur TRANSGENE (EPA:TNG)
Transgene: major advances and outlook for 2025
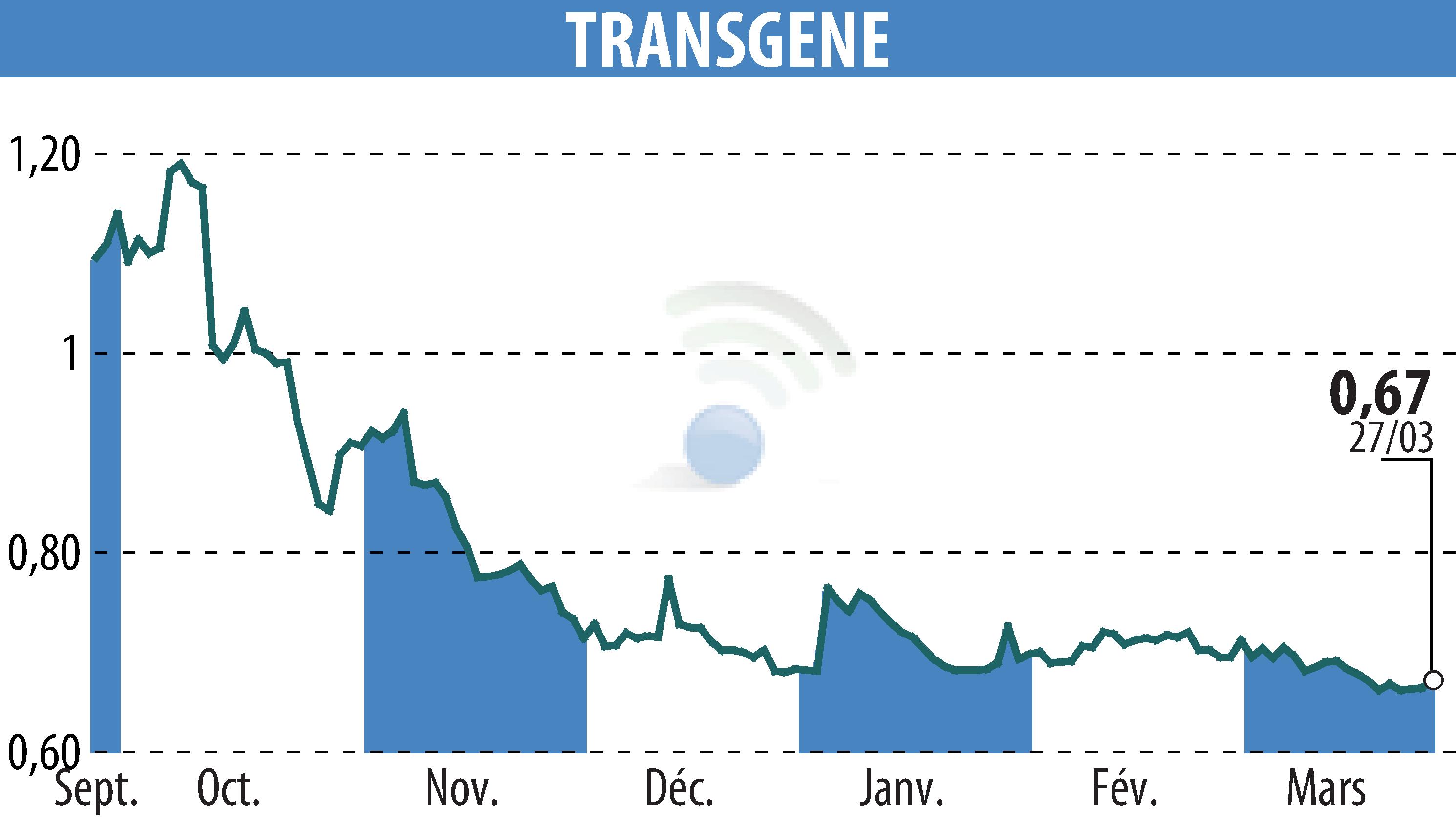
Strasbourg-based biotechnology company Transgene made significant progress in 2024 with its individualized therapeutic vaccine TG4050. This vaccine, intended for patients with head and neck cancers, achieved Phase I clinical proof of principle, revealing a positive immune response in treated subjects. In 2025, Transgene intends to expand its clinical trials, with a Phase II portion already underway, aimed at confirming the encouraging results obtained initially. The company also plans to launch a new trial in another indication by the end of 2025.
Financially, Transgene is posting a net loss of €34 million for 2024, partly due to increased research and development spending. However, thanks to restructured financing, the company is assured of resources until the end of April 2026, allowing it to focus on its strategic ambitions and the development of its portfolio of innovative immunotherapies.
R. E.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de TRANSGENE
