sur THERACLION (EPA:ALTHE)
Theraclion Successfully Completes Pivotal Study with 96.8% Occlusion Rate
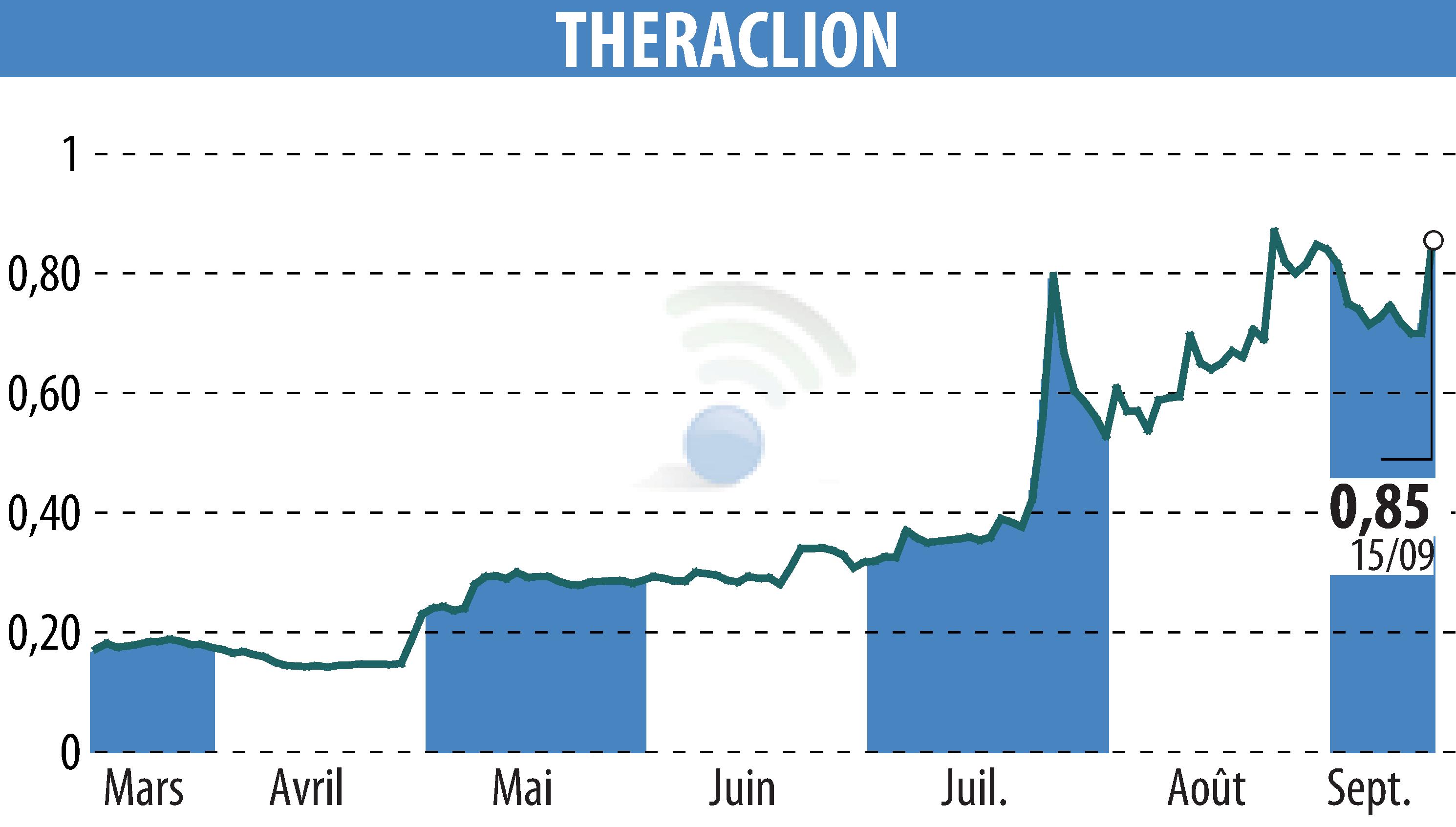
Theraclion announced the results of its VEINRESET study, involving Sonovein®, its non-invasive varicose vein treatment platform. With an occlusion rate of 96.8% at 12 months, the clinical trial demonstrated strong efficacy. Only one mild adverse event was reported, highlighting an excellent safety profile.
The reflux abolition rate reached 98.5%, demonstrating the effectiveness of high-intensity focused ultrasound (HIFU). The study enrolled 70 patients at various centers, including New York and Vienna. The results will inform the FDA's application for approval, paving the way for access to the US market.
Patients reported a reduction in pain and symptoms. Investigators expressed satisfaction with the results, promising a reliable, non-invasive alternative to existing treatments.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de THERACLION
