sur Tharimmune Inc. (NASDAQ:THAR)
Tharimmune Reports Positive Phase 1 Results for TH104 at ACG Meeting
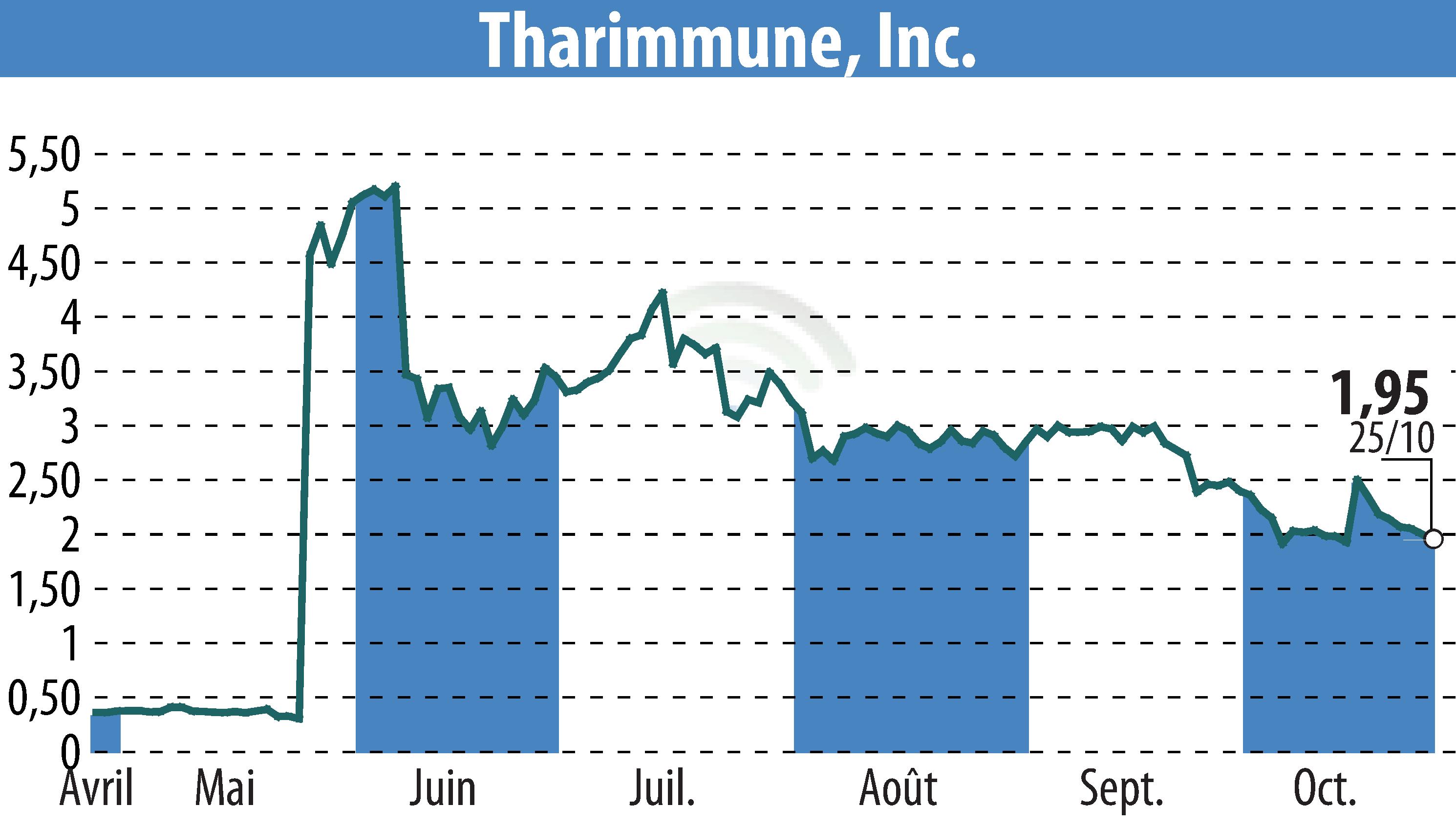
Tharimmune Inc., a clinical-stage biotechnology company, has presented favorable Phase 1 data for their lead candidate, TH104, at the 2024 American College of Gastroenterology meeting. TH104 is being developed for moderate-to-severe pruritus in chronic liver disease. The trial revealed no opioid withdrawal effects, underscoring its safety profile.
The Phase 1 trial was a single-dose study conducted on patients with chronic liver disease. Primary measures focused on safety and tolerability. TH104 showed promising effects with a 33.3% mean reduction in pruritus scores 24 hours post-dosing. The study reported no significant adverse events, paving the way for further clinical trials.
Tharimmune anticipates beginning a Phase 2 trial soon to further explore TH104's efficacy in primary biliary cholangitis patients. They expect topline results by 2025, engaging with both U.S. and EU regulators as part of their development strategy.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Tharimmune Inc.
