sur SANOFI-AVENTIS (EPA:SAN)
Sanofi's Rezurock Recommended for EU Approval by CHMP
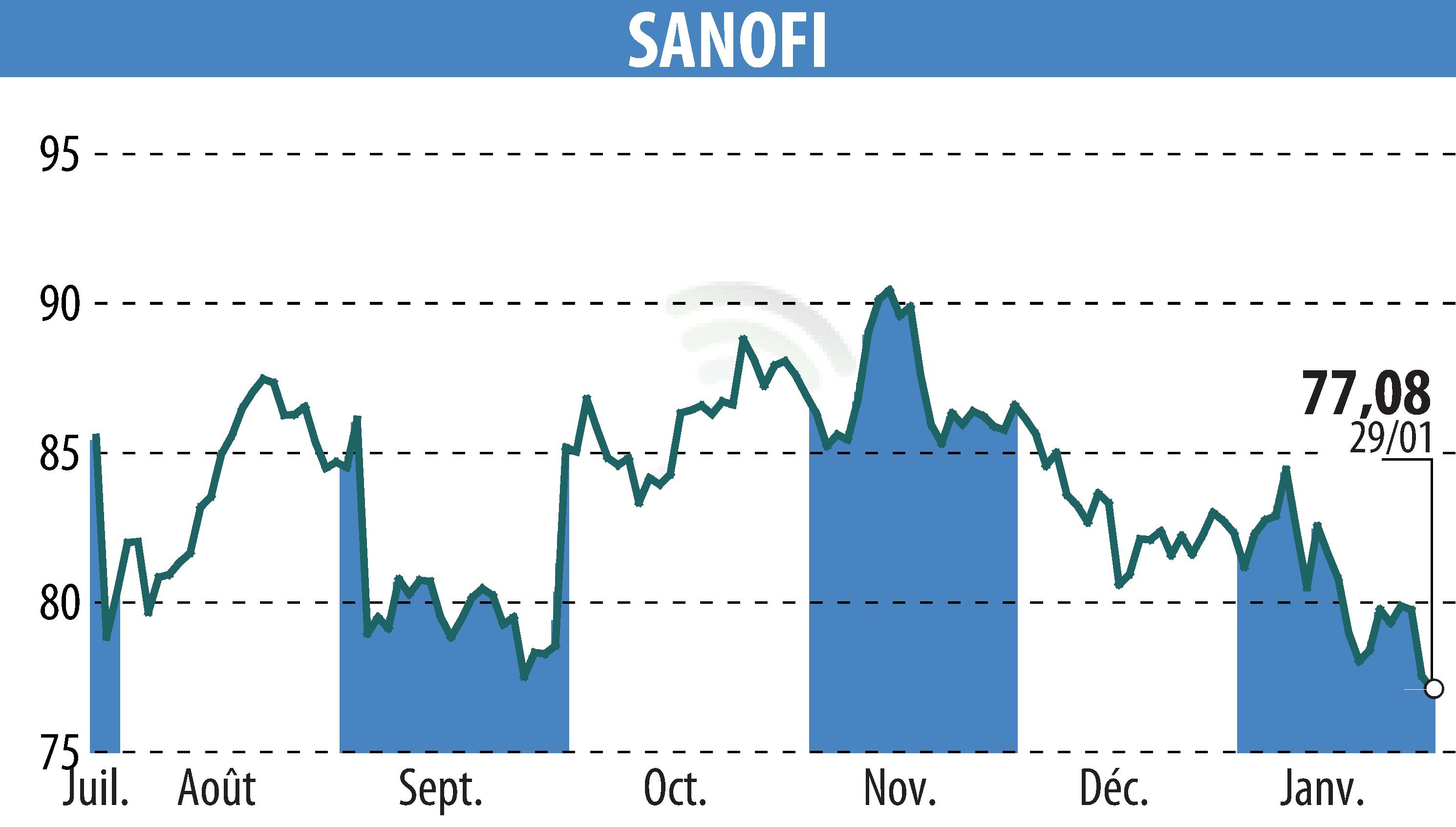
Sanofi's Rezurock, a treatment for chronic graft-versus-host disease (GVHD), has been recommended for conditional EU approval by the Committee for Medicinal Products for Human Use (CHMP). This follows a positive opinion from the European Medicines Agency, supporting its safety and efficacy from multiple studies and real-world evidence. If sanctioned, Rezurock would offer a new option for both adults and children aged 12 and older suffering from late-line chronic GVHD.
This recommendation is significant, following Sanofi's appeal against a previous negative opinion by the CHMP in October 2025. It is backed by data from the phase 2 ROCKstar study, which showed promising results.
Rezurock is already approved in over 20 countries, including the U.S. and Canada, and this positive CHMP opinion could mean its EU launch is imminent once approved by the European Commission.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
