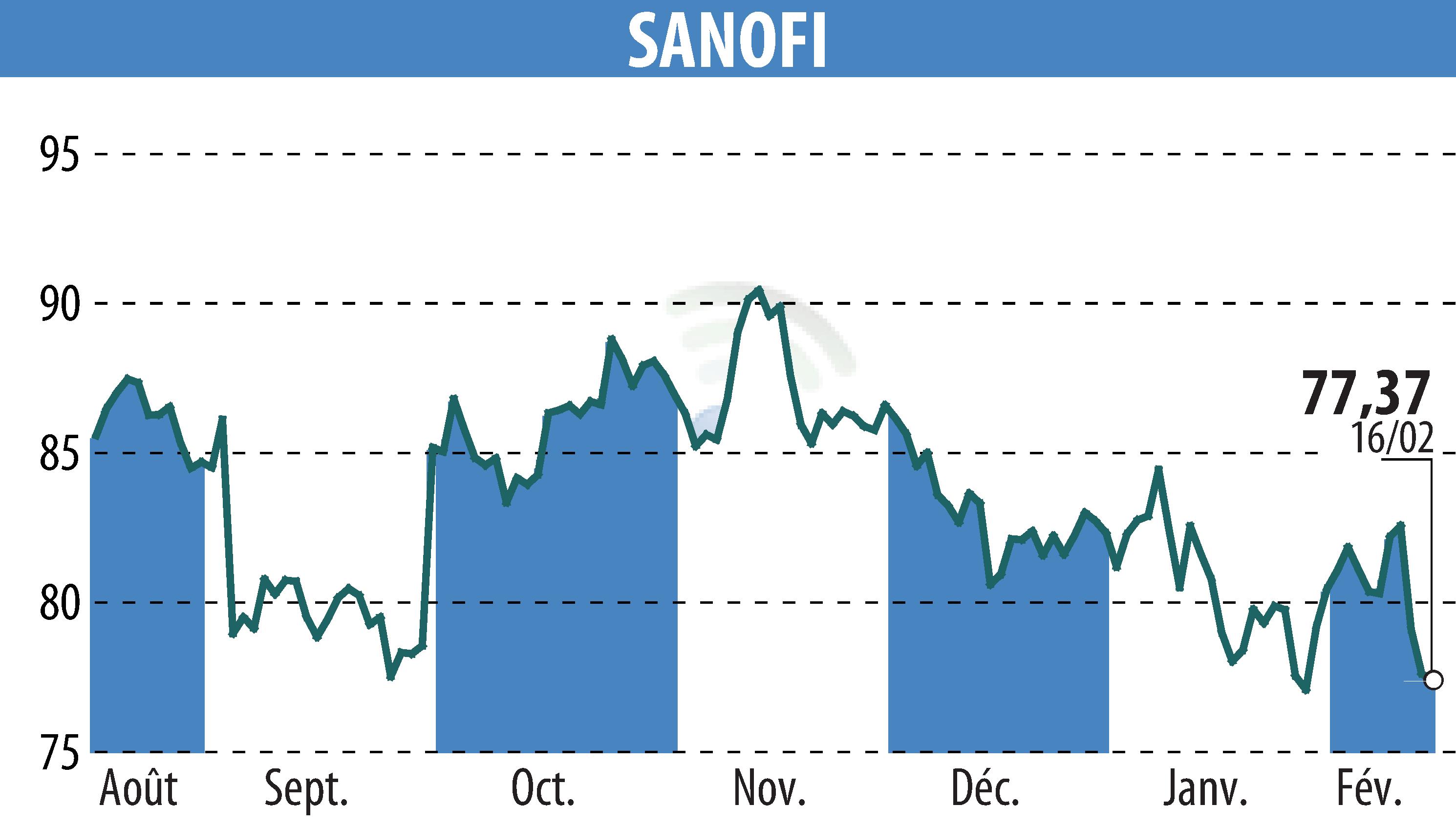
sur SANOFI-AVENTIS (EPA:SAN)
Sanofi and Teva Report Positive Phase 2b Data for Duvakitug in IBD
Sanofi and Teva's phase 2b study on duvakitug reveals promising results for ulcerative colitis and Crohn’s disease. The RELIEVE UCCD LTE study demonstrated that duvakitug maintains clinical and endoscopic efficacy over 44 weeks for patients initially responsive after a 14-week induction phase.
The study highlights duvakitug's potential as an effective treatment for inflammatory bowel disease (IBD), marking significant progress for patients with few options. Response rates at week 44 showed 58% (900 mg) and 47% (450 mg) remission for UC, and 55% (900 mg) and 41% (450 mg) endoscopic response for CD.
Duvakitug was well tolerated, with adverse events consistent with previous studies. These findings underscore duvakitug’s viability as a therapy, with ongoing phase 3 trials set to explore further potential. Sanofi leads its global development while Teva oversees commercialization in specific regions.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
