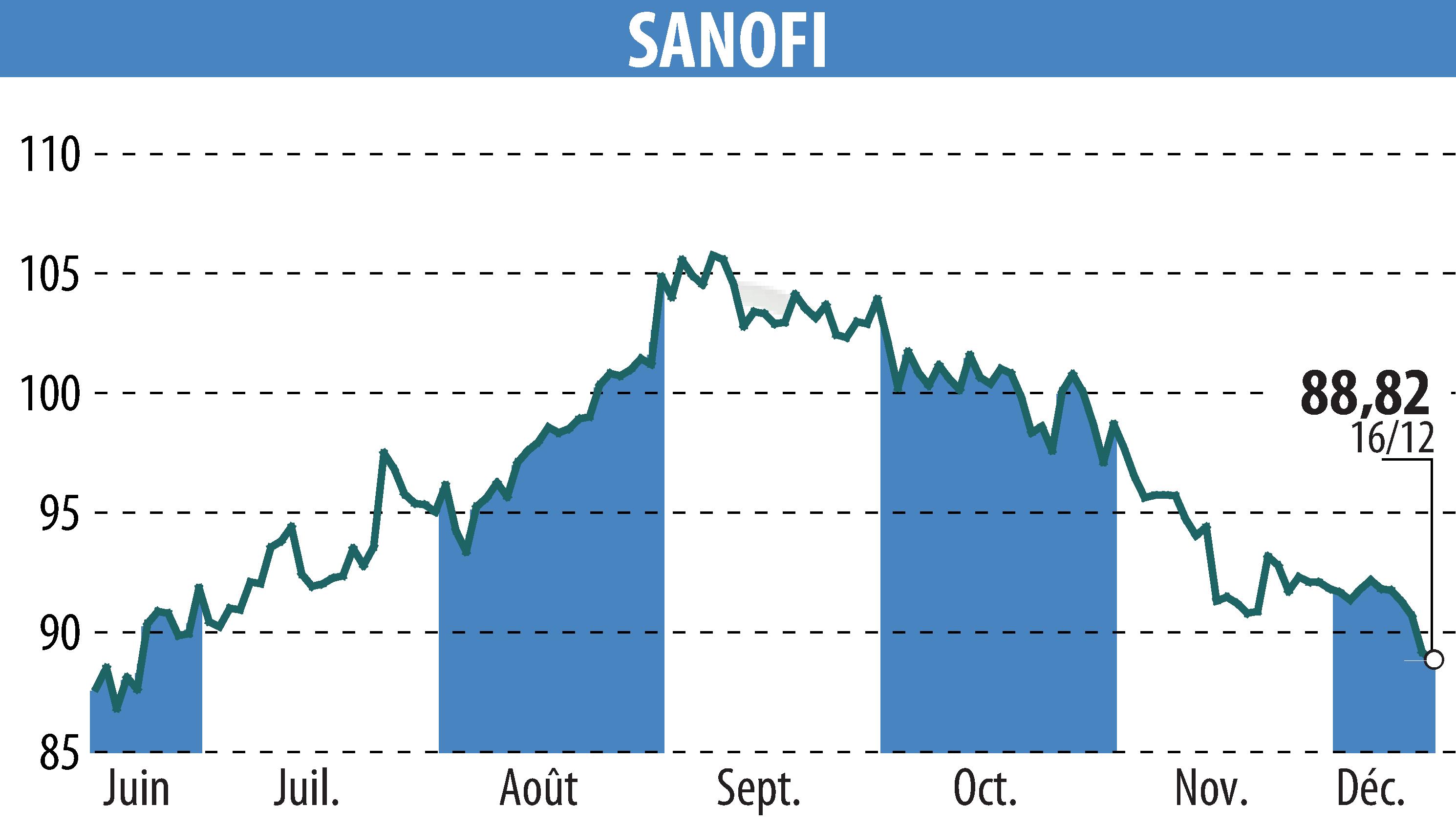
sur SANOFI-AVENTIS (EPA:SAN)
Sanofi and Teva Announce Promising Phase 2b Results for Duvakitug
Sanofi and Teva Pharmaceuticals have released encouraging results from their phase 2b RELIEVE UCCD study, indicating the potential of duvakitug, a monoclonal antibody, in treating ulcerative colitis (UC) and Crohn’s disease (CD). The study met its primary endpoints, showing significant clinical remission and endoscopic response in UC and CD patients, respectively.
In UC, 47.8% of high-dose patients achieved clinical remission, starkly higher than the 20.45% on placebo. CD patients similarly benefited, with 47.8% reaching an endoscopic response compared to 13% on placebo. The absence of significant safety signals supports the drug’s tolerability.
Pending regulatory discussions, Sanofi and Teva plan to commence phase 3 trials. These results bolster Sanofi's and Teva's commitment to advancing innovative treatments for inflammatory bowel disease.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
