sur Protagonist Therapeutics, Inc. (NASDAQ:PTGX)
Protagonist Therapeutics Reports Positive Phase 3 Trial Results for Icotrokinra in Psoriasis
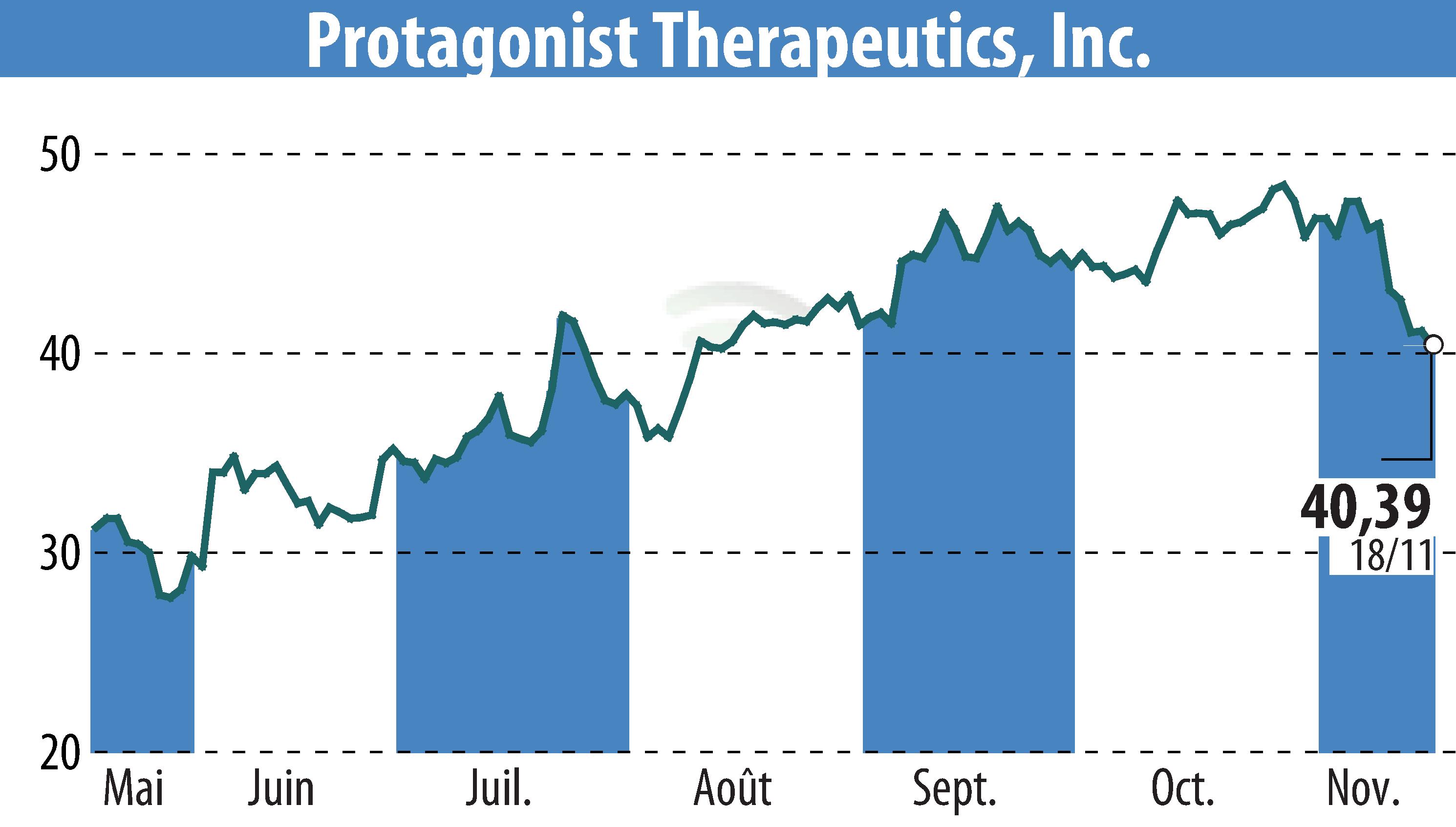
Protagonist Therapeutics has announced promising results from Phase 3 studies of icotrokinra, a targeted oral peptide for plaque psoriasis. The trials, ICONIC-LEAD and ICONIC-TOTAL, both met primary endpoints, highlighting icotrokinra's effectiveness in achieving significant skin clearance. At week 24, 74% of patients achieved nearly clear skin.
The studies, conducted with Johnson & Johnson, confirm icotrokinra's potential as an innovative oral treatment option with a favorable safety profile. Protagonist has earned $165 million in milestone payments following these successful outcomes and expects further payments as development continues.
Protagonist plans to present detailed results at upcoming medical congresses and anticipates initiating new studies. The company remains on track to potentially submit a New Drug Application in 2025.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Protagonist Therapeutics, Inc.
