sur IPSEN (EPA:IPN)
New analysis of the CONTACT-02 trial by Ipsen
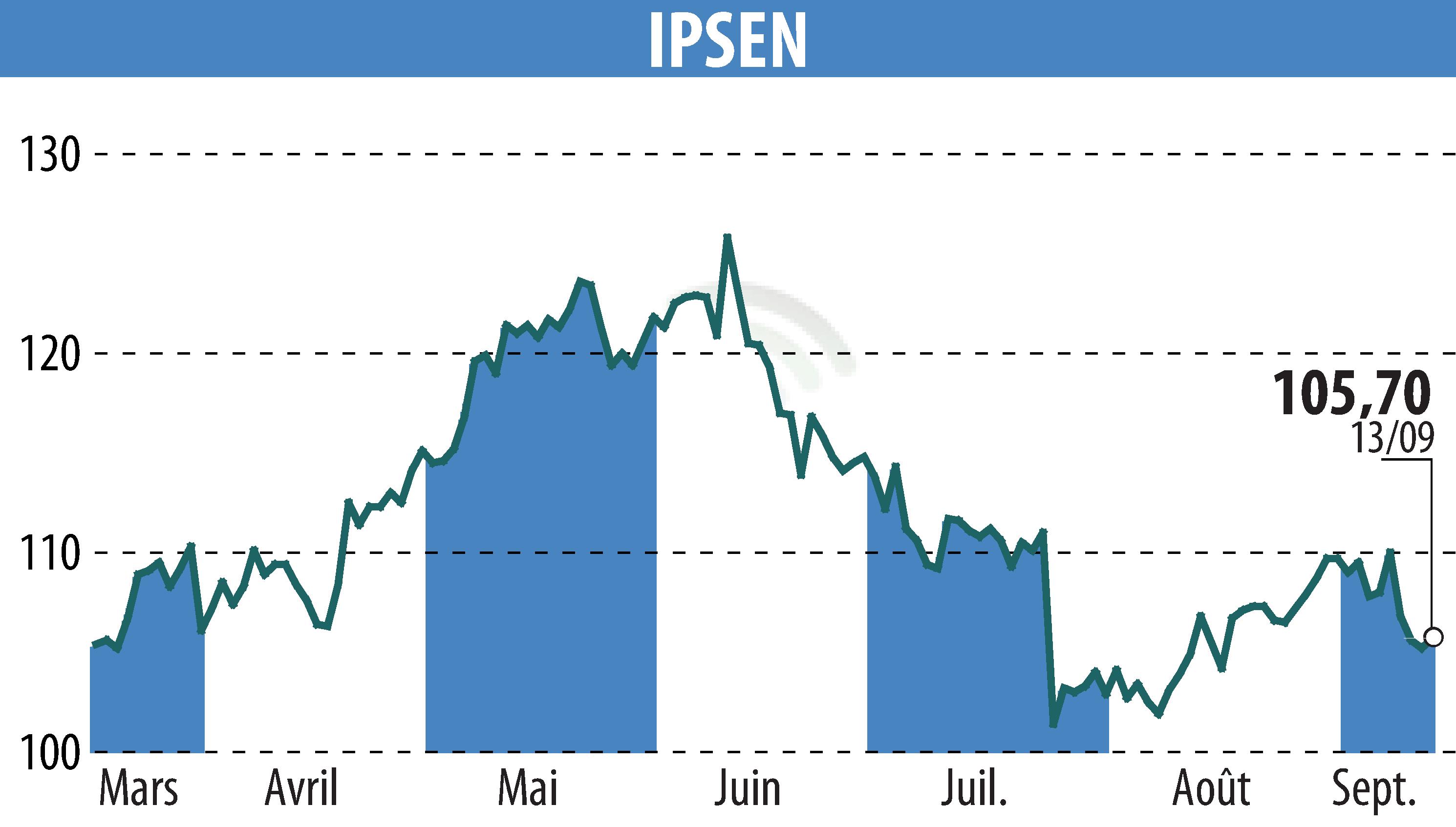
Ipsen has announced final results from the Phase III CONTACT-02 trial of Cabometyx® (cabozantinib) in combination with atezolizumab for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Although an improvement in overall survival was observed, the results did not reach statistical significance.
As a result, Ipsen will not seek regulatory approval for this combination therapy in territories where it has marketing rights, excluding the United States and Japan. However, the company remains confident in the efficacy of Cabometyx as a monotherapy and in combination with immunotherapy for existing and future indications.
The CONTACT-02 study, conducted on 575 patients, met another primary endpoint, progression-free survival, with a statistically significant result. The safety profile of the combination treatment was consistent with expectations, with no new safety issues identified.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de IPSEN
