sur NEOVACS (EPA:ALNEV)
Neovacs Addresses Rumors About PXT3003
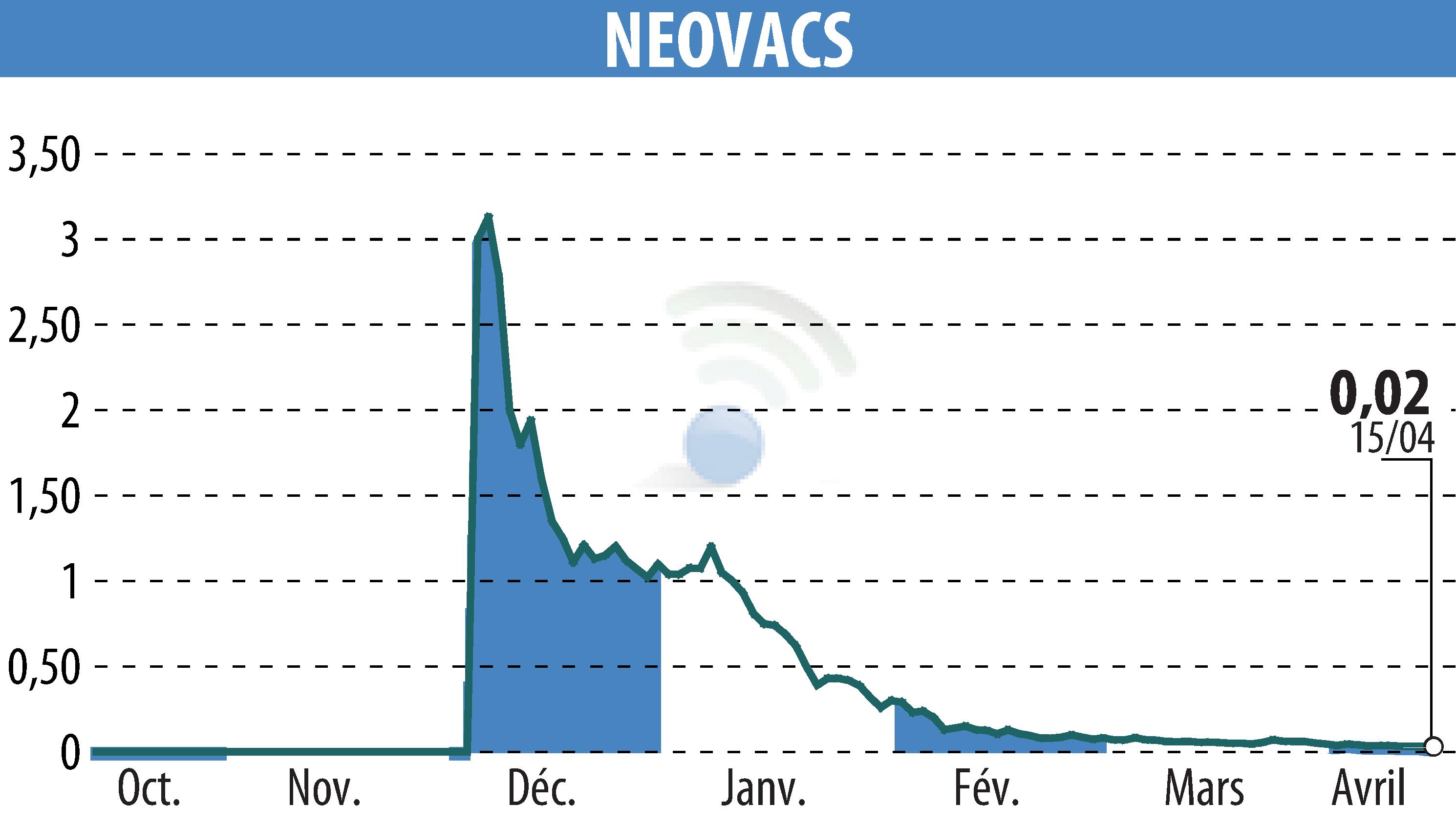
On April 16, 2025, Neovacs, a biopharmaceutical company, addressed rumors surrounding PXT3003, a drug candidate for Charcot-Marie-Tooth disease type 1A. This drug was originally developed by Pharnext, which had licensed the drug for China to a joint venture majority-owned by Tasly. Recently, it was reported that Tasly has applied for marketing authorization in China for this drug. Neovacs cannot currently confirm this information but is in contact with its Chinese partner for clarification.
If approved in China, PXT3003 could benefit Neovacs' assets and potentially support approval applications in other markets. However, the company emphasizes the uncertainty surrounding the outcome of this procedure, having already recorded a full provision for the claim in its accounts by mid-2024.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de NEOVACS
