sur NanoViricides, Inc. (NASDAQ:NNVC)
NanoViricides Investigates NV-387 for MPOX Treatment Under WHO's MEURI Protocol
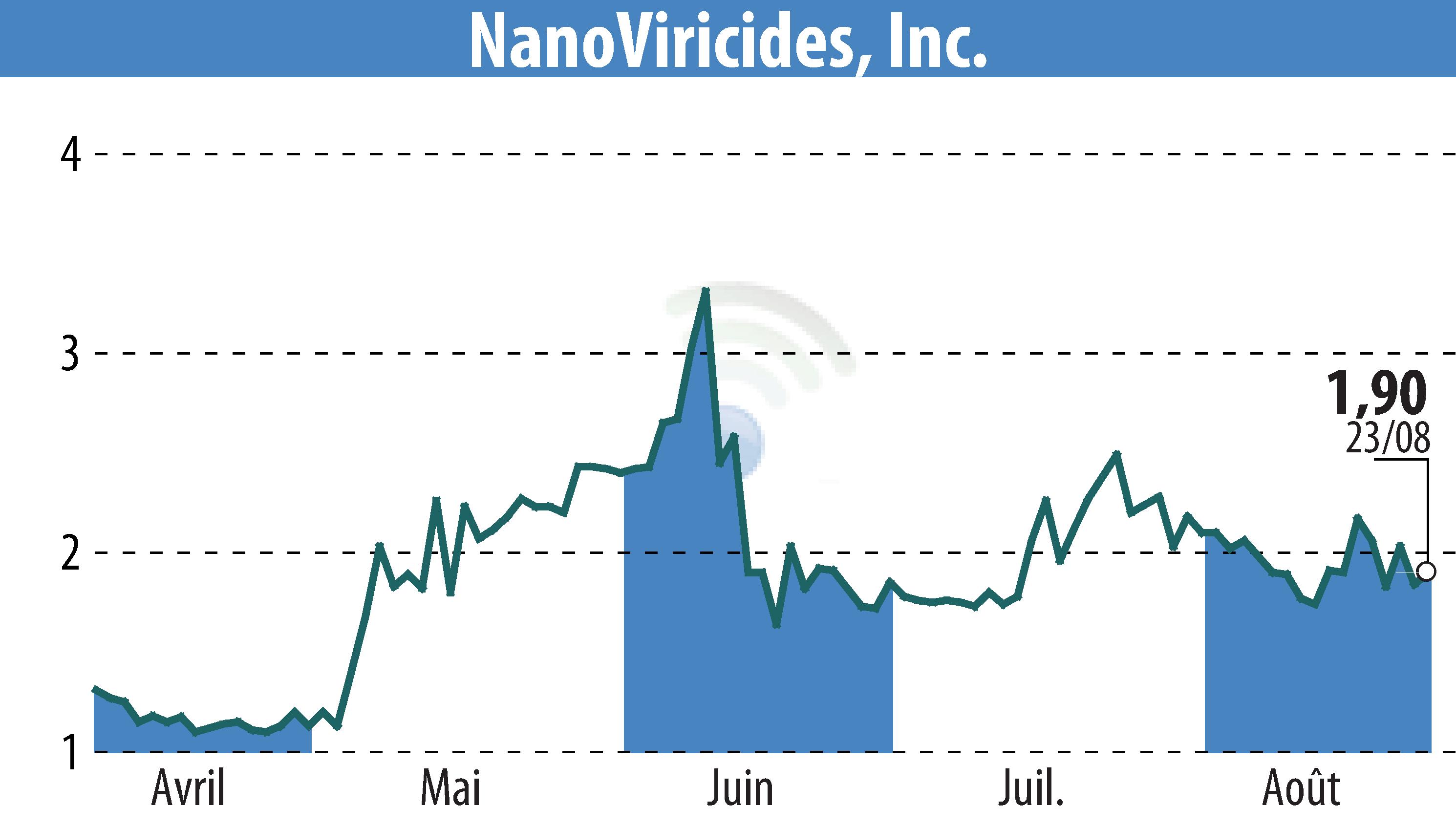
SHELTON, CT / ACCESSWIRE / August 26, 2024 / NanoViricides, Inc. (NYSE American:NNVC) is exploring the use of NV-387 for treating MPOX patients under the WHO's MEURI protocol. This investigation follows the WHO's declaration of the 2024 MPOX outbreak as a Public Health Emergency of International Concern, spreading into over ten African countries and cases emerging in Thailand and Sweden.
NV-387 has passed Phase I clinical trials showing excellent safety and tolerability. It has demonstrated significant improvements in survival rates in animal models compared to tecovirimat. Notably, NV-387-m-T formulation showed a 112.5% improvement in survival versus the 75% improvement of tecovirimat in the study.
The Company suggests NV-387's broad-spectrum antiviral properties might revolutionize viral infection treatments if proven effective in human trials. The current MPOX outbreak primarily involves Clade 1b, which spreads faster and is more severe than Clade 2, highlighting a need for new treatments.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de NanoViricides, Inc.
