sur NanoViricides, Inc. (NASDAQ:NNVC)
NanoViricides Advances NV-387 to Phase II Clinical Trial
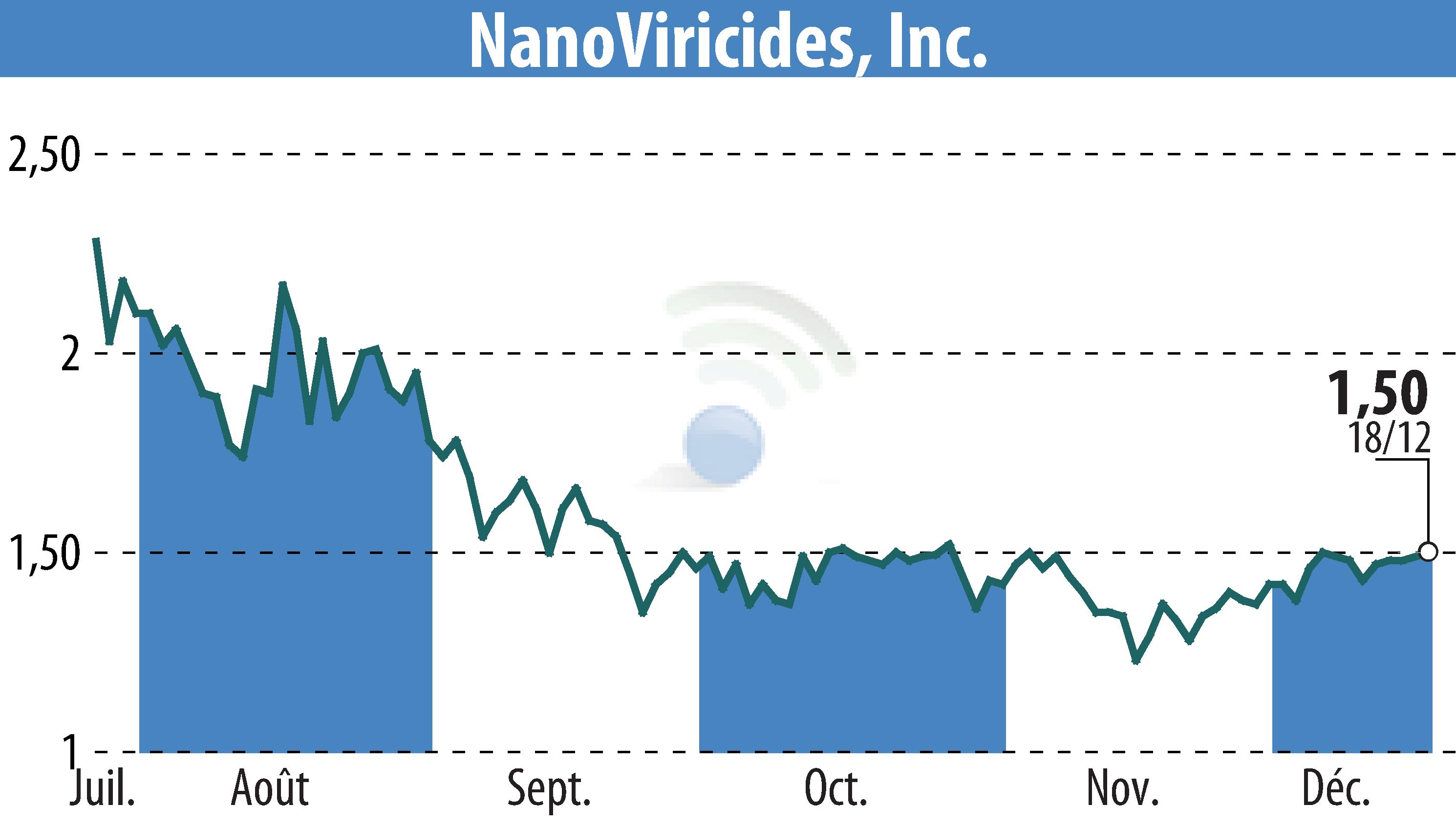
NanoViricides, Inc., based in Shelton, CT, has partnered with a Clinical Research Organization to initiate a Phase II clinical trial for its antiviral drug NV-387. This advancement marks an important step in the regulatory journey of NV-387, a broad-spectrum antiviral designed to treat viral diseases, including MPox.
NV-387 aims to address the MPox disease, prevalent in regions like the Democratic Republic of Congo and Uganda, where no effective drug currently exists. The World Health Organization declared a Public Health Emergency of International Concern regarding MPox in August 2024.
Animal models have shown NV-387's significant antiviral activity against orthopoxviruses, suggesting potential effectiveness against both MPox and Smallpox. The drug is designed to prevent virus escape by mimicking cellular structures viruses bind to, making viral mutation escape unlikely.
R. E.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de NanoViricides, Inc.
