sur NANOBIOTIX (EPA:NANO)
NANOBIOTIX Provides Business Update and Reports Half Year 2024 Financial Results
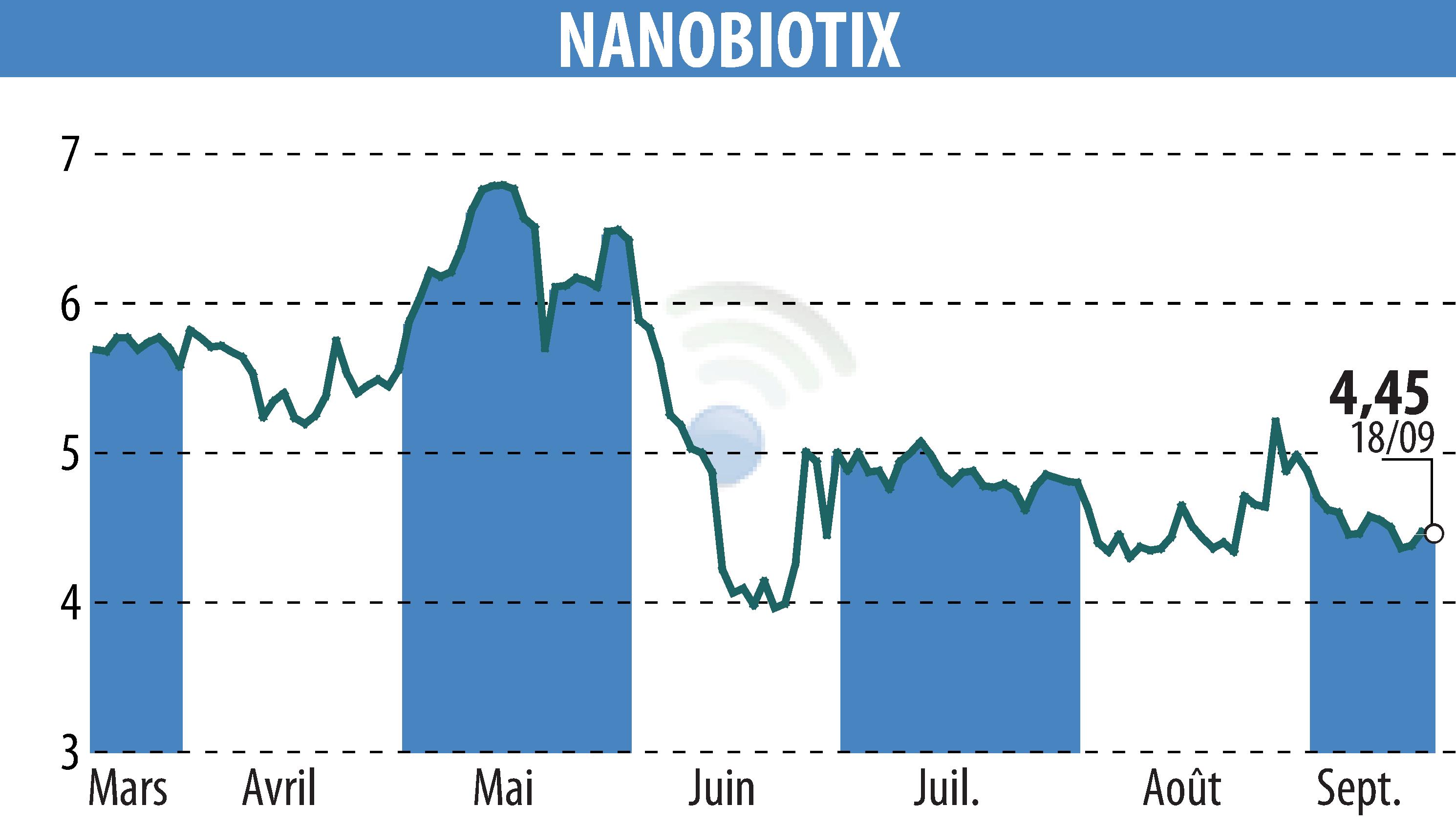
NANOBIOTIX has announced progress and financial results for the first half of 2024. The lead candidate NBTXR3, under a licensing agreement with Janssen Pharmaceutica NV, showed a favorable safety profile and tumor response in patients. The company expects several clinical milestones in the next 12-18 months, including updated and initial data in pancreatic, head and neck, esophageal, and lung cancers.
Operational highlights include nominations to the Supervisory Board and the achievement of a milestone in the NANORAY-312 trial. Additionally, various clinical trials are progressing, such as those in non-small cell lung cancer and head and neck cancers.
Financially, the company reported an increase in revenue to €9.3 million and a net loss of €21.9 million, down from €28.1 million the previous year. Cash and equivalents stood at €66.3 million, extending the cash runway to Q4 2025.
R. H.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de NANOBIOTIX
