sur Monogram Technologies Inc. (NASDAQ:MGRM)
Monogram Technologies Advances mBôs TKA System with Regulatory Updates and Clinical Trials in India
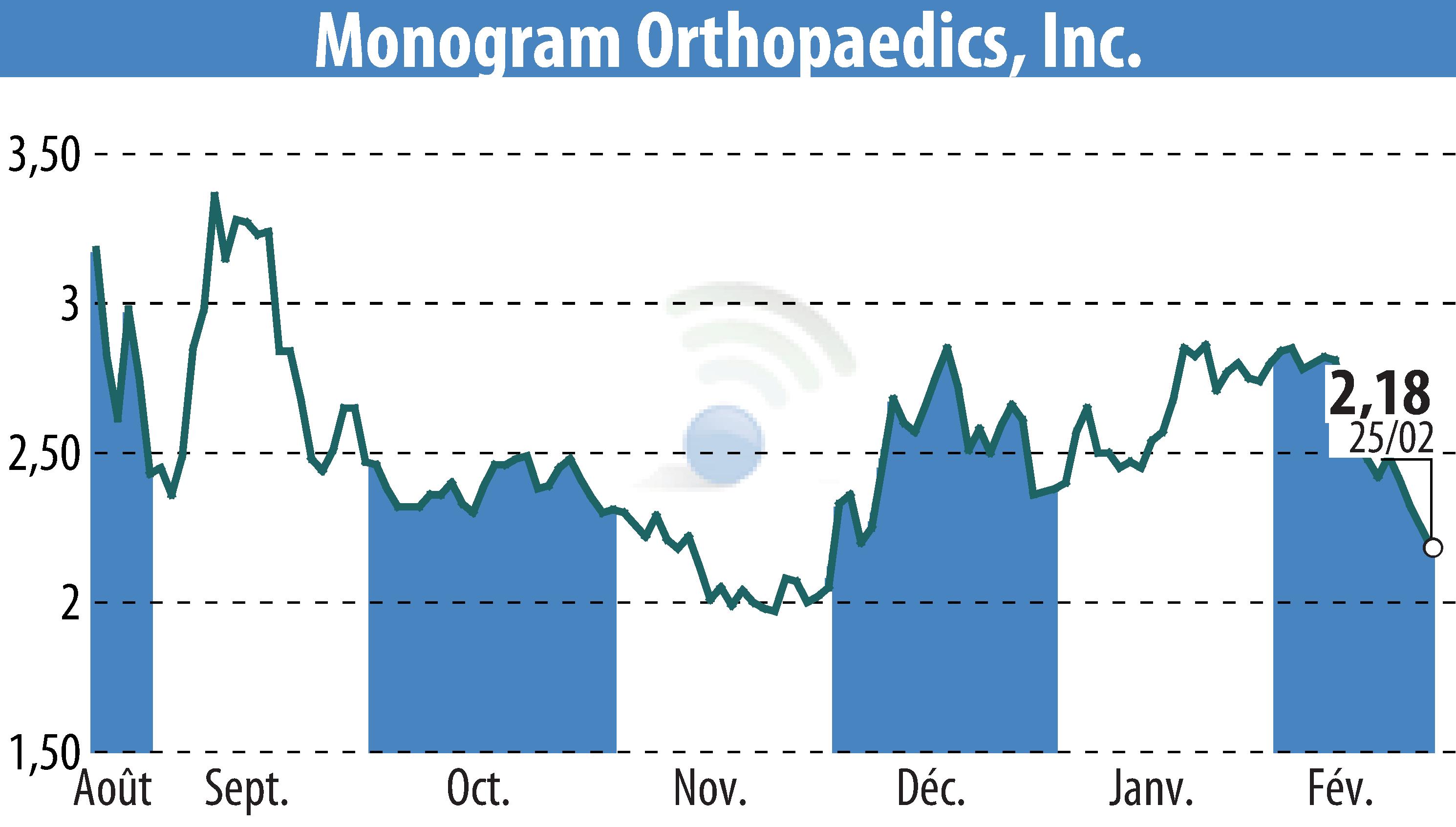
Monogram Technologies Inc. has finalized all supplemental testing for its mBôs TKA System and submitted a formal response to the FDA's Additional Information Request. The company expects an FDA clearance decision to follow, potentially allowing U.S. commercialization.
In collaboration with Shalby Limited, Monogram plans a multicenter clinical trial in India. The trial preparation included an Investigator Meeting and training at Shalby Hospital in Ahmedabad, facilitated by Reliance Life Sciences, a key regulatory sponsor.
Monogram highlights significant advancements, notably a cutting system enabling a 300% increase in feed rate while maintaining precision. The company's aim is a robotic system competitive with manual surgery times.
CEO Ben Sexson emphasizes the importance of speed and safety in surgery, asserting confidence in Monogram's progress toward an effective autonomous system. The company's dedication reflects in its rigorous testing process and ongoing innovation in orthopedic robotics.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Monogram Technologies Inc.
