sur MIRA Pharmaceuticals (NASDAQ:MIRA)
MIRA Pharmaceuticals Targets Neuropathic Pain with Ketamir-2, Submits Pre-IND Request to FDA
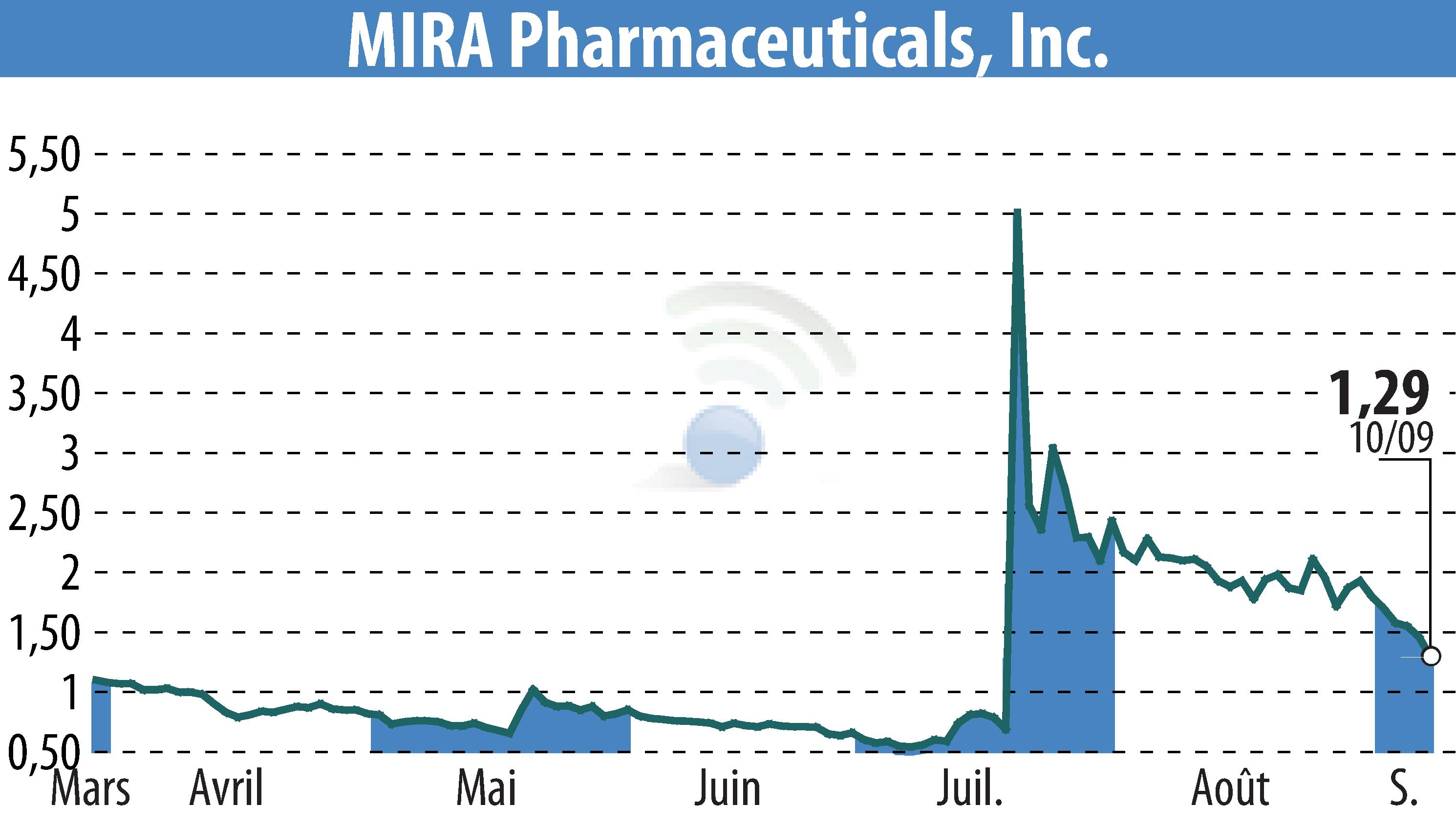
MIRA Pharmaceuticals, Inc. (NASDAQ:MIRA), a preclinical-stage firm, announced it has designated neuropathic pain as the primary indication for its novel oral ketamine analog, Ketamir-2. The company has submitted a pre-Investigational New Drug (IND) meeting request to the U.S. Food and Drug Administration (FDA), with the meeting anticipated in November 2024. MIRA plans to submit its IND by year-end, aiming for human trials in Q1 2025.
Neuropathic pain is a complex condition often caused by nervous system damage. The North American market for its treatments is projected to reach $5.2 billion by 2030. Current treatments often provide limited relief and significant side effects. MIRA's Ketamir-2 aims to improve therapeutic efficacy and safety compared to existing options.
Ketamir-2 is designed as a low-affinity NMDA receptor antagonist that selectively binds to the phencyclidine site. Unlike opioids, it is classified as a non-controlled substance, making it more accessible. Preclinical studies suggest it offers consistent pain relief without typical sedative effects, positioning Ketamir-2 as a promising candidate in CNS disorder treatment.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MIRA Pharmaceuticals
