sur MIRA Pharmaceuticals (NASDAQ:MIRA)
MIRA Pharmaceuticals Submits IND for Ketamir-2 to FDA
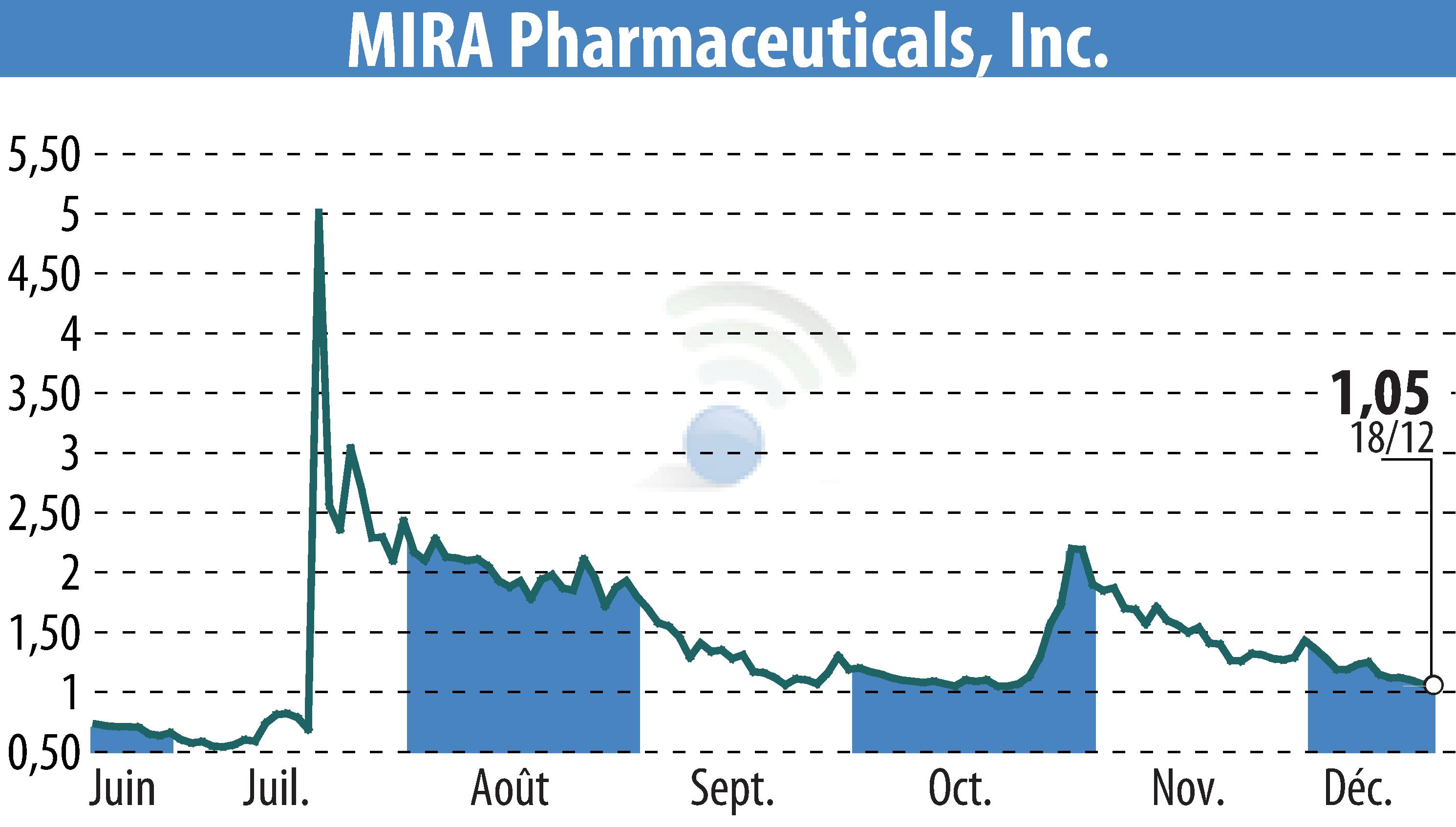
MIRA Pharmaceuticals, Inc. has submitted an Investigational New Drug (IND) application to the FDA for Ketamir-2. This marks a significant step for its potential treatment of neuropathic pain. The company aims to advance its lead drug candidate by beginning Phase I trials in early 2025.
Ketamir-2 is an oral ketamine analog targeting neuropathic pain and other neuropsychiatric conditions. It promises reduced side effects compared to existing treatments by selectively targeting the NMDA receptor. It is notably not classified as a controlled substance, simplifying regulatory hurdles.
The neuropathic pain market is projected to grow significantly by 2030. Current treatments like gabapentin and opioids have limitations, which Ketamir-2 aims to overcome. Upcoming clinical trials will assess its safety and efficacy.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MIRA Pharmaceuticals
