sur MIRA Pharmaceuticals (NASDAQ:MIRA)
MIRA Pharmaceuticals Pushes Forward with Ketamir-2 Development, Targeting Early Clinical Efficacy in 2025
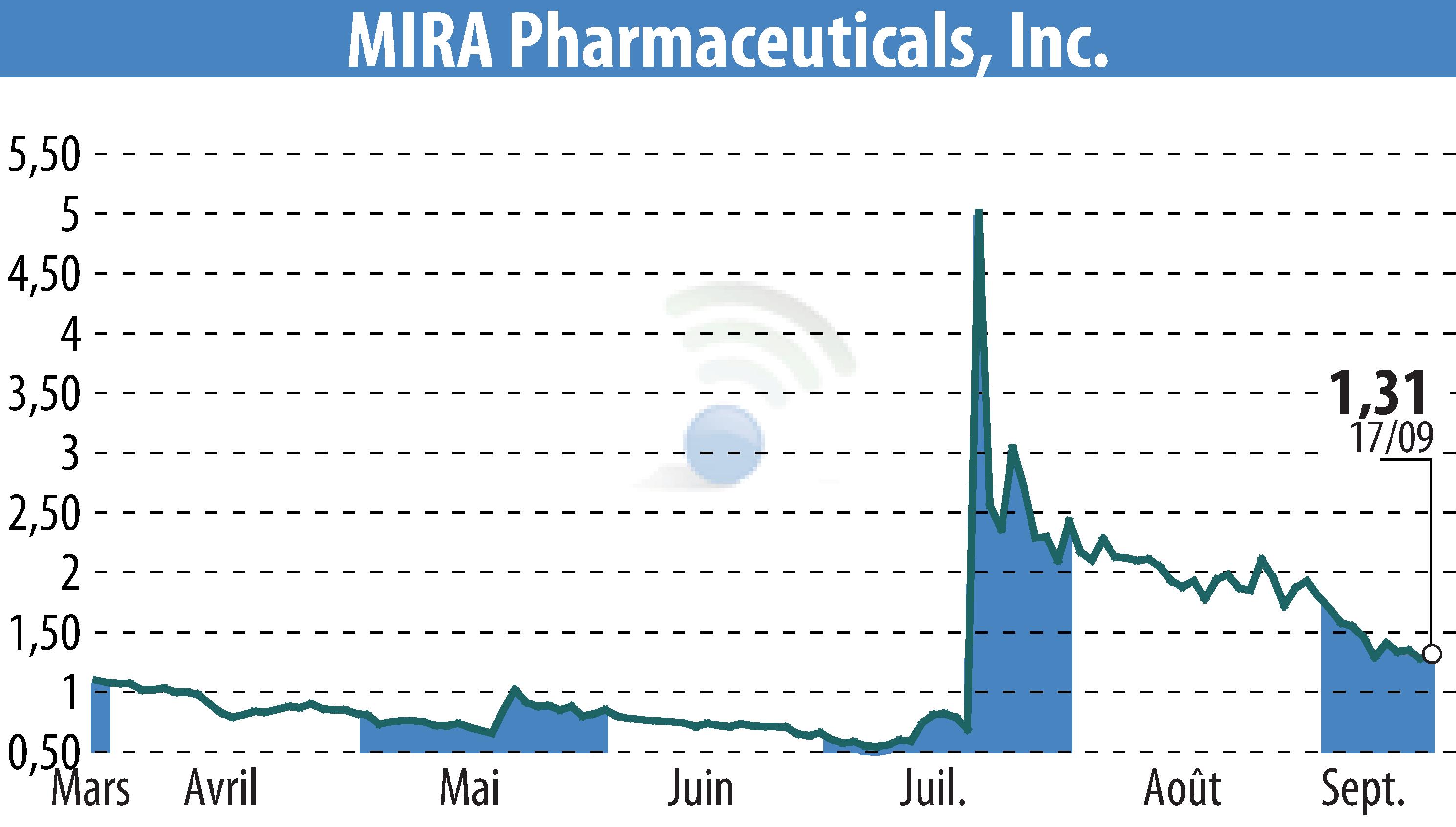
MIRA Pharmaceuticals, Inc. (NASDAQ:MIRA) has announced advances in the development of its novel oral ketamine analog, Ketamir-2. The company aims to demonstrate clinical efficacy as early as 2025 through strategic Phase I/II study designs.
In collaboration with Formulex, MIRA is working on drug product development, focusing on optimizing oral bioavailability of Ketamir-2 capsules. The Investigational New Drug (IND) filing with the FDA is on schedule for December 2024.
Preclinical studies in animal models of Diabetic Neuropathy and Cancer-Induced Neuropathy are ongoing, with results expected by the end of 2024. The Phase I clinical trial design has been finalized and is set to begin in early Q1 2025, assessing safety, tolerability, and pharmacokinetics.
MIRA is exploring additional indications in mental health, including major depressive disorder and PTSD. These initiatives aim to provide early and robust clinical data, positioning Ketamir-2 as a potential treatment for neuropathic pain and other conditions.
R. E.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MIRA Pharmaceuticals
