sur MIRA Pharmaceuticals (NASDAQ:MIRA)
MIRA Pharmaceuticals Advances Towards Clinical Trials with Ketamir-2
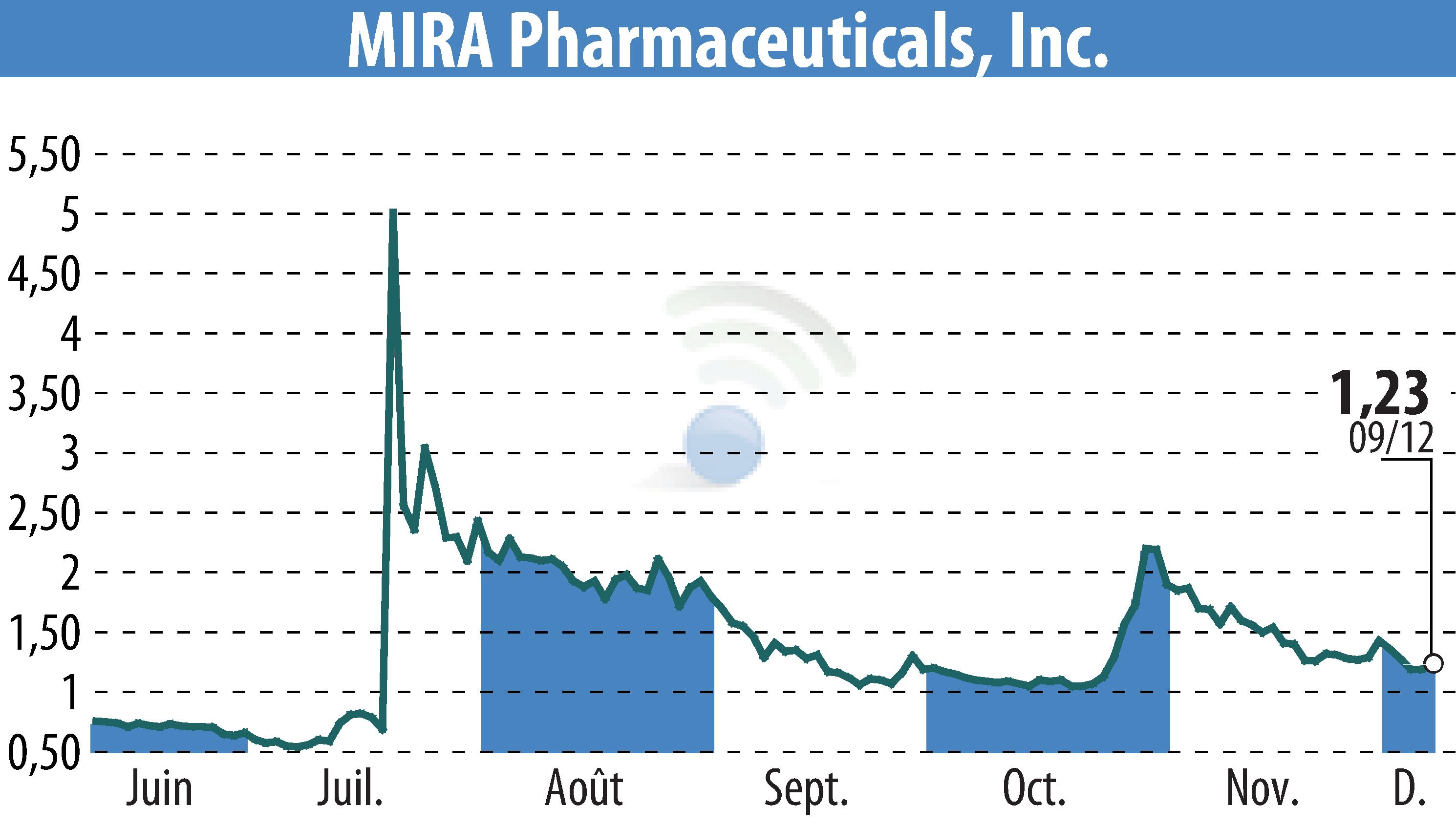
MIRA Pharmaceuticals, Inc. has successfully completed its preclinical safety program for Ketamir-2, a new oral ketamine analog. Validation of its safety profile marks a significant milestone, as the company prepares to submit its Investigational New Drug (IND) application by the end of 2024.
The absence of adverse findings positions MIRA to advance into clinical trials, focusing on neuropathic pain. This development is crucial as nearly 40% of drug failures result from safety issues. Comprehensive studies demonstrated Ketamir-2's safety across various assessments, including cardiovascular and CNS evaluations.
Phase I trials, beginning in early 2025, aim to assess safety, tolerability, and pharmacokinetics in healthy participants. MIRA anticipates starting Phase IIa trials later that year, aligning with its strategic plan for growth and partnership.
R. H.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MIRA Pharmaceuticals
