sur MEDINCELL (EPA:MEDCL)
Medincell: Promising advances for injectable Olanzapine
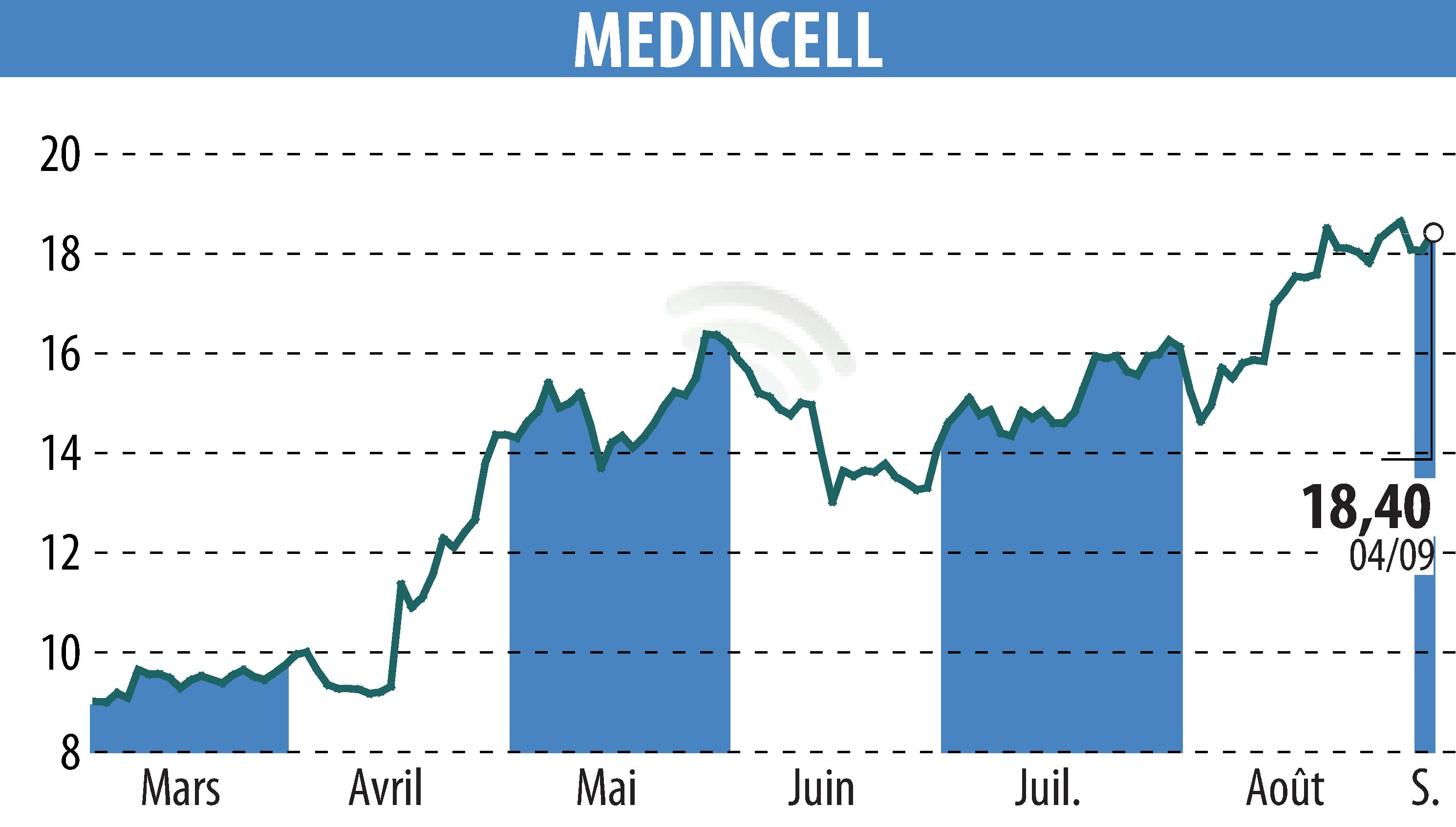
Teva Pharmaceuticals, in collaboration with Medincell, announced significant progress in the Phase 3 clinical trial for Olanzapine Long-Acting Injection (LAI). Approximately 99% of injections planned for approval were completed without the observation of post-injection sedation syndrome (PDSS).
Full safety data, essential for approval, are expected in the second half of 2024. According to results published in May 2024, the efficacy of Olanzapine LAI has been confirmed.
This treatment, administered monthly, could become the first without an FDA "black box" warning for PDSS, unlike other existing Olanzapine formulations. Teva is responsible for its development and marketing.
Medincell could receive up to $117 million in development and commercialization milestones for this program, in addition to royalties on net sales.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MEDINCELL
