sur Jaguar Health (NASDAQ:JAGX)
Jaguar Health's Crofelemer Shows Promising Results for Chronic Diarrhea
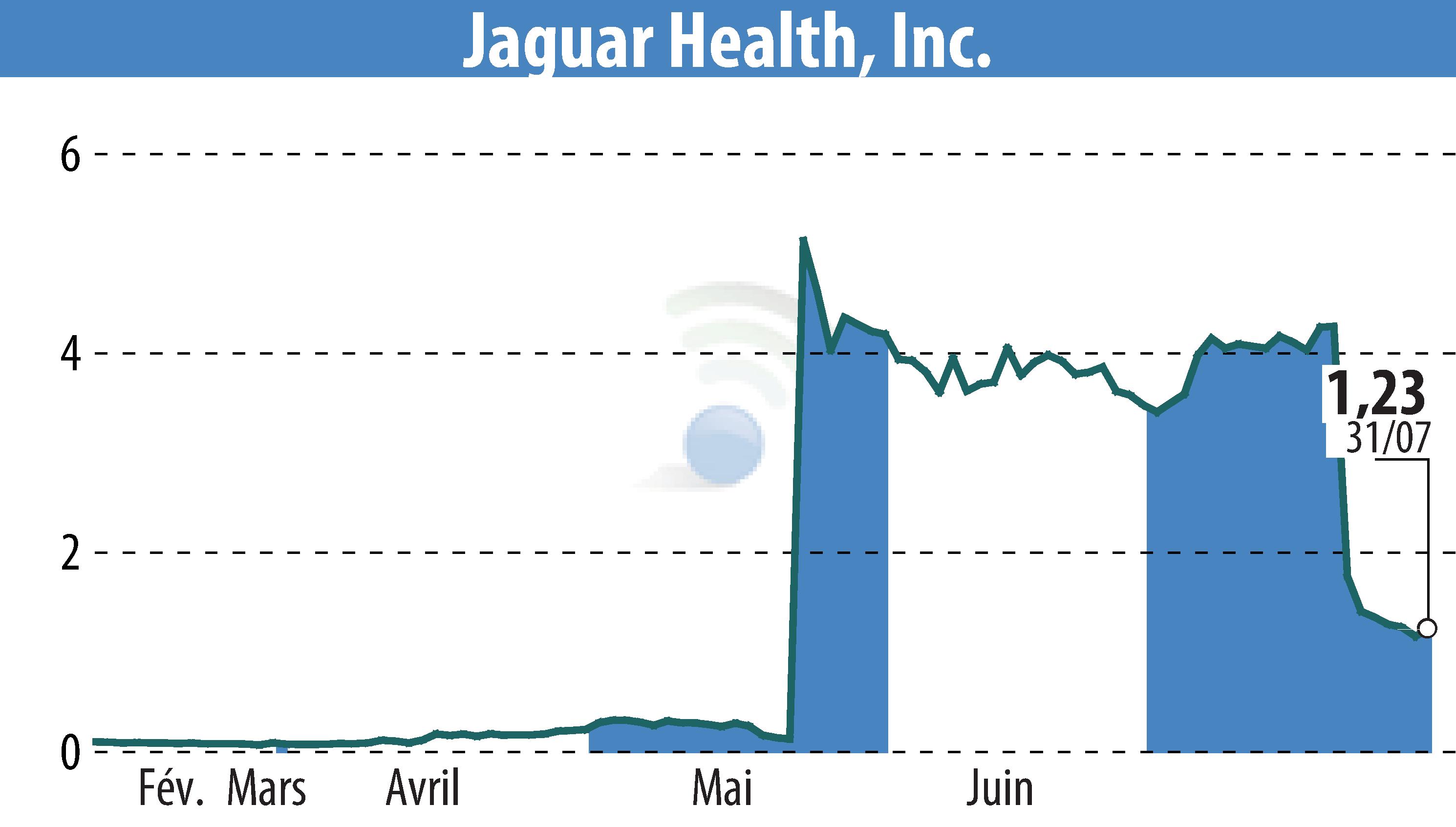
Jaguar Health, Inc. (NASDAQ:JAGX) announced positive outcomes from two independent studies of crofelemer, Napo Pharmaceuticals' novel drug, for managing chronic refractory diarrhea. Results will be presented at the American College of Gastroenterology Annual Scientific Meeting in October 2024.
The first study, led by Dr. Judy Nee at Beth Israel Deaconess Medical Center, showed that crofelemer significantly improved stool consistency and reduced abdominal pain in patients with functional diarrhea. Notably, the drug did not cause constipation.
The second study, led by Dr. Brooks D. Cash at the University of Texas Health Science Center, found that nearly 60% of patients with chronic idiopathic diarrhea experienced symptom relief from crofelemer. This pilot study suggests the need for larger trials to further validate these findings.
Functional diarrhea affects patients for over six months without effective treatments. Chronic idiopathic diarrhea impacts 3-5% of people in developed countries, significantly reducing quality of life and adding economic burdens.
Crofelemer, derived from the Croton lechleri tree, is the only FDA-approved drug under botanical guidelines, highlighting its unique plant-based origin.
R. H.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health
