sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Submits Study on Diarrhea in Breast Cancer Patients
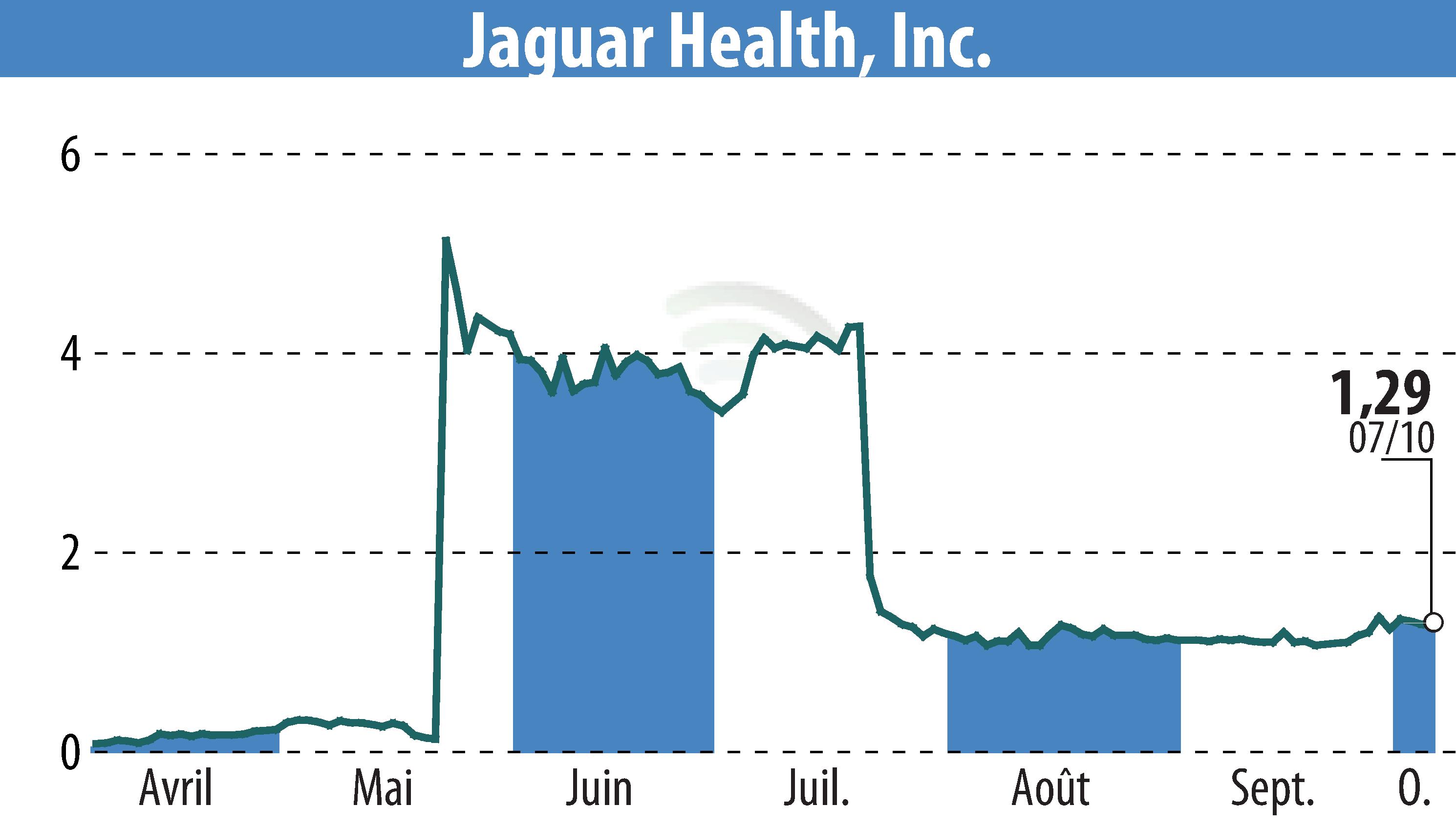
Jaguar Health, Inc. has submitted a compelling abstract for a study examining the impact of diarrhea from targeted cancer therapies on breast cancer patients. This analysis focuses on a cohort from the placebo group in the OnTarget phase 3 trial, conducted by Napo Pharmaceuticals, a subsidiary of Jaguar. Among the 142 participants with solid tumors, nearly 75 had breast cancer and were undergoing targeted therapies with or without standard chemotherapy.
The study presents unique data from patient-reported outcomes using digital platforms, providing insight into gastrointestinal symptoms such as diarrhea, abdominal pain, urgency, and fecal incontinence. These findings offer valuable perspectives on necessary cancer therapy dose modifications due to therapy-related diarrhea.
Napo Pharmaceuticals plans further analysis and discussions with the FDA, underlining the importance of patient-centric studies to enhance supportive care for affected patients.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
