sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health's Napo Pharmaceuticals Seeks FDA Guidance on Crofelemer for Rare Pediatric Disease
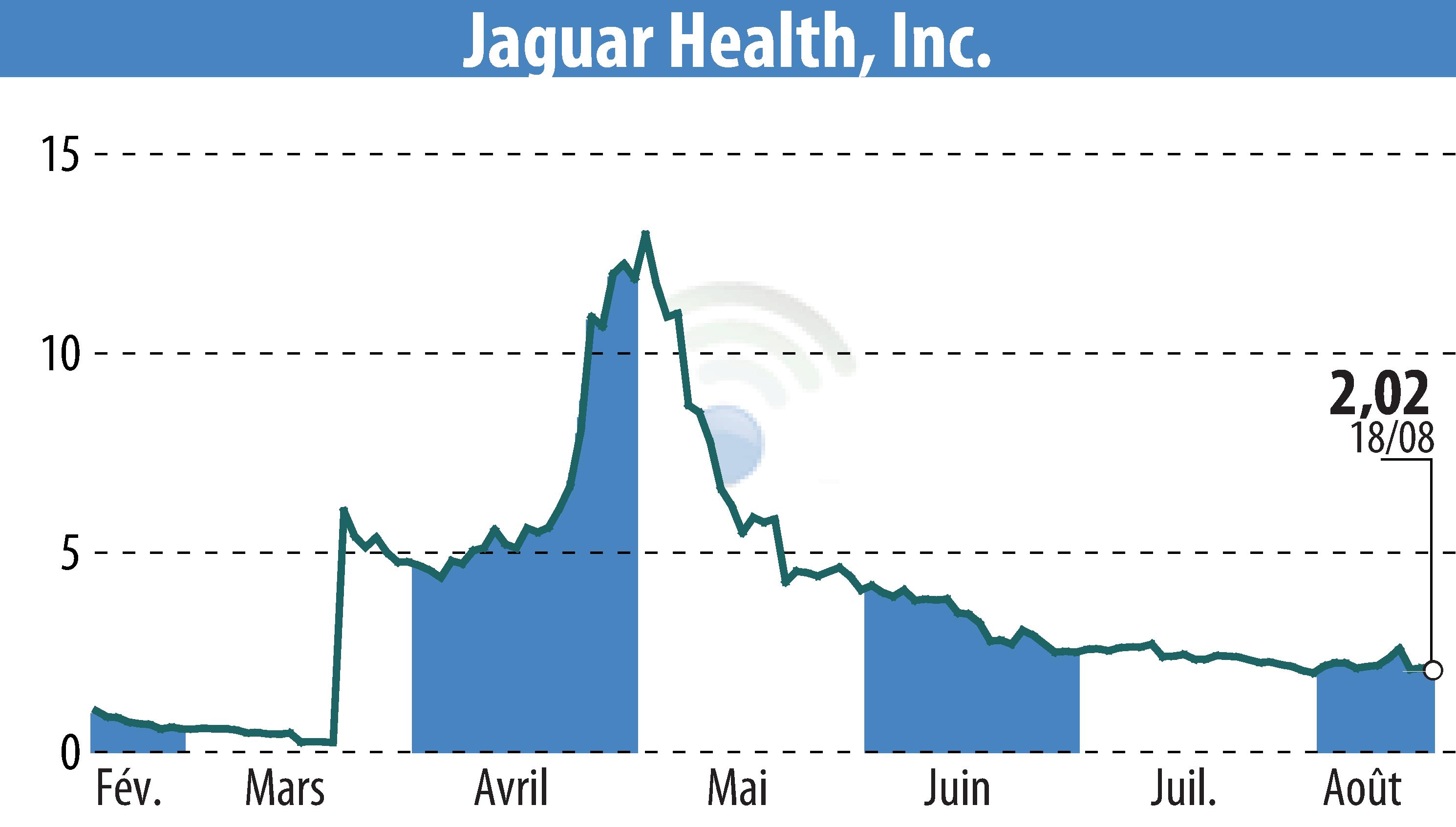
Jaguar Health, through its subsidiary Napo Pharmaceuticals, is set to meet with the U.S. FDA to discuss regulatory pathways for crofelemer, aimed at treating microvillus inclusion disease (MVID). This pediatric disorder currently lacks approved drug treatments, and Napo is keen on exploring expedited regulatory options. The company's ongoing clinical trials show promising initial results, with crofelemer reducing parenteral nutrition needs by 27% in one patient.
The promising data from Abu Dhabi will be presented at the 2025 NASPGHAN Annual Meeting in Chicago. Jaguar supports ongoing investigator-initiated trials and is conducting further studies across multiple regions under regulatory guidance. If successful, crofelemer could emerge as a novel therapeutic option, potentially reducing complications associated with MVID.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
