sur Heidelberg Pharma AG (isin : DE000A11QVV0)
Heidelberg Pharma AG's Interim Report for First Nine Months of 2024
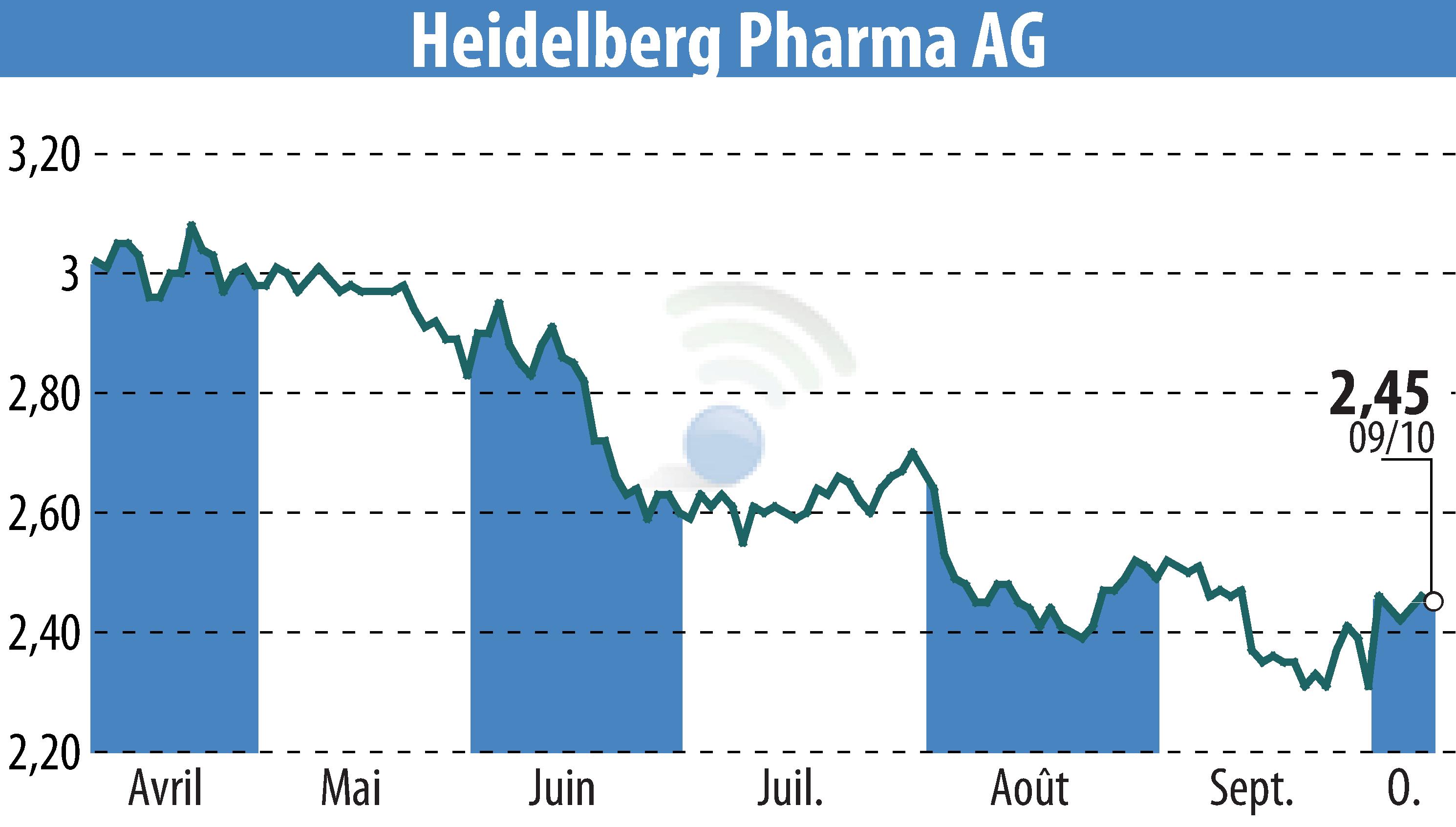
Heidelberg Pharma AG has released its interim management statement outlining key achievements and financial outcomes for the first nine months of 2024. The company's HDP-101 clinical trial in Europe and the US continues, with adjustments to the protocol for dose optimization. Notably, a new clinical finding revealed complete remission in one patient at the International Myeloma Society's annual meeting.
The firm also sold a portion of future royalties for TLX250-CDx to HealthCare Royalty, boosting finances despite overall decreased revenue. Sales and income reached EUR 7.6 million, down from EUR 13.9 million the previous year. Operating expenses were EUR 22.8 million, a significant reduction from EUR 30 million.
Guidance has been adjusted due to lower R&D costs and higher revenue projections. The updated financial outlook estimates sales of EUR 10-12 million and a reduced cash requirement, ensuring financial security until mid-2025.
R. E.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Heidelberg Pharma AG
