sur Nanohale AG (isin : DE000A1EWVY8)
Formycon Reports Continued Growth in 2024 Nine-Month Results
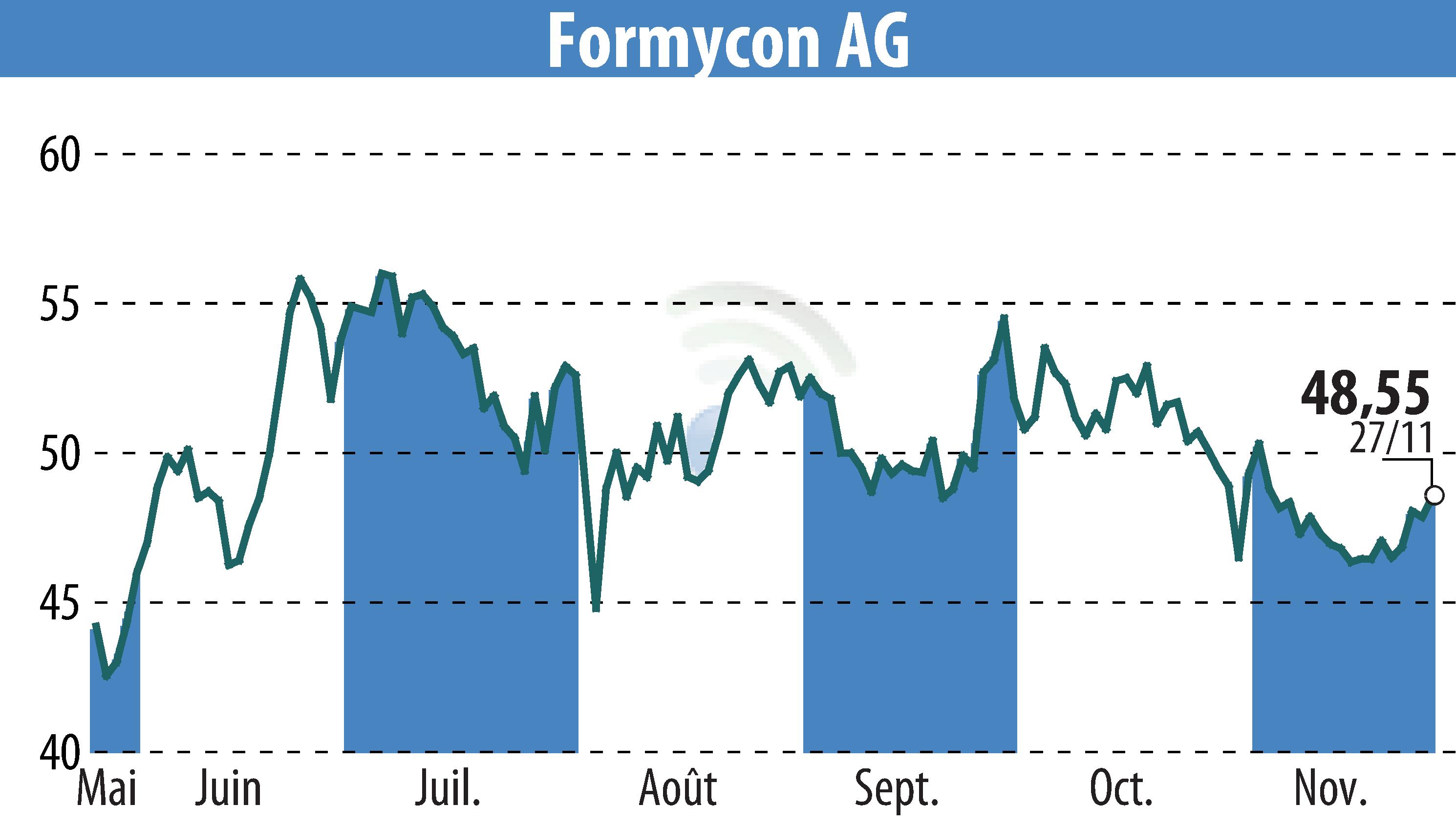
Formycon AG announced promising results for the first nine months of 2024. The company experienced notable successes with product approvals for its biosimilars, FYB202 in the US and Europe, and initiated clinical trials for the Keytruda® biosimilar candidate FYB206. Formycon's strategy is validated by its financial performance, meeting its guidance.
The company’s ranibizumab biosimilar, FYB201, gained a strong market position in the US and UK. Additionally, the FDA and European Commission approved the FYB202 biosimilar for severe inflammatory diseases, setting the stage for its market launch by early 2025. The development pipeline expanded with the launch of FYB210, indicating future growth in the immunology sector.
Formycon's revenue for the period was approximately €41.1 million, with the adjusted EBITDA reflecting strong FYB201 performance. The recent uplisting to the Prime Standard of the Frankfurt Stock Exchange marks a strategic milestone, enhancing Formycon's visibility and investor appeal on the global stage.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Nanohale AG
