sur Jaguar Health, Inc. (NASDAQ:JAGX)
FDA Renews Approval for Canalevia-CA1, Jaguar Health's Canine Diarrhea Drug
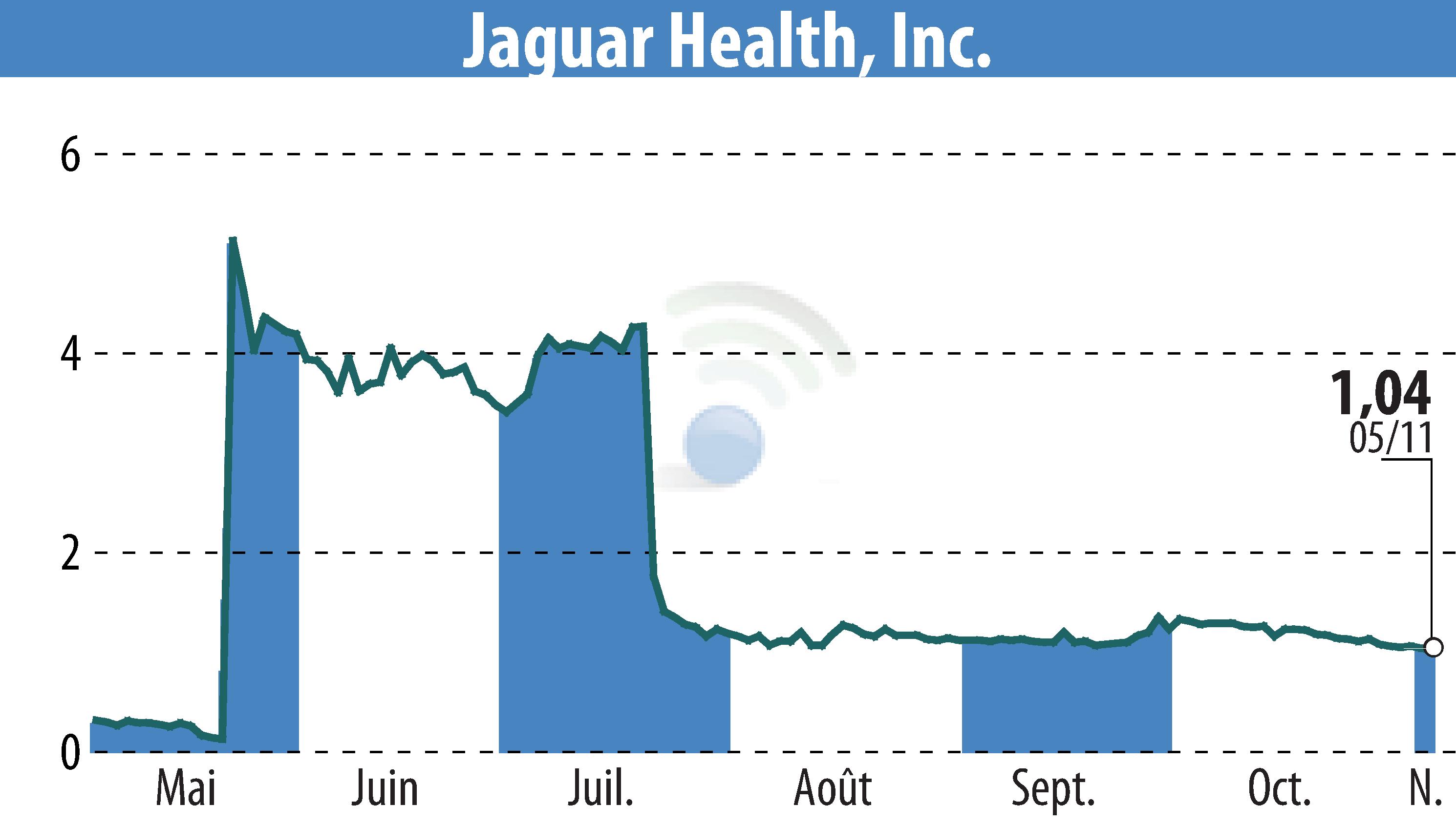
The FDA has renewed its conditional approval for Canalevia-CA1, Jaguar Health's novel treatment for chemotherapy-induced diarrhea (CID) in dogs. Canalevia-CA1 is the only FDA-approved product in this category and remains available through several veterinary distributors, such as Chewy, until December 2025.
The renewal acknowledges Jaguar's ongoing efforts to prove the drug's full efficacy. Dr. Michael Guy from Jaguar emphasized the importance of Canalevia-CA1 for dogs suffering from CID, marking a vital step toward full drug approval. CID is a significant side effect of cancer treatment in dogs, impacting their quality of life and treatment continuity.
Canalevia-CA1, derived from the Croton lechleri tree, remains the sole plant-based oral prescription product approved. Jaguar Health is committed to improving animal welfare through innovative treatments.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
