sur EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical's Q3 2024 Financials and Upcoming FDA Milestones
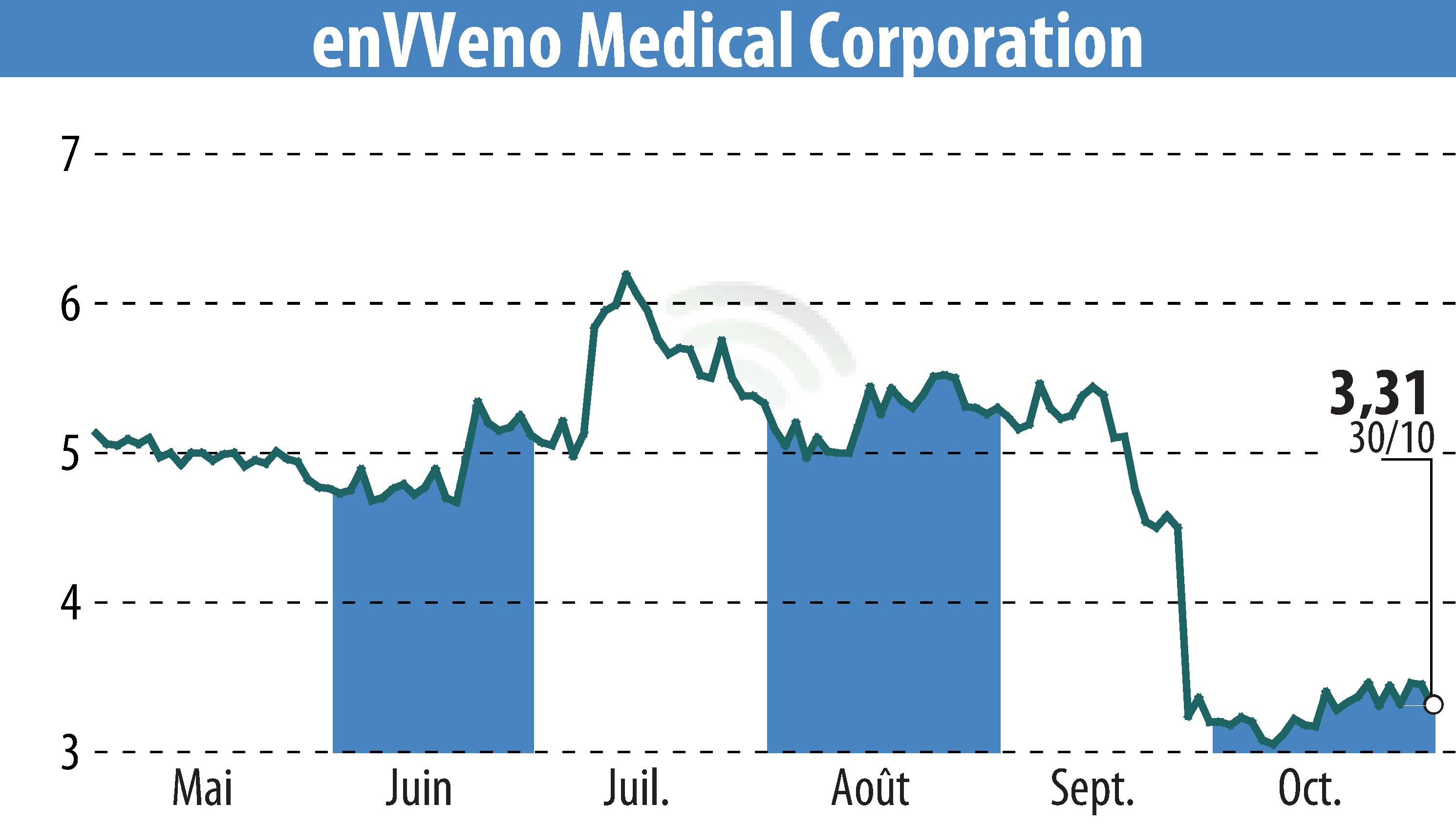
enVVeno Medical Corporation, known for its advancements in venous disease treatment, announced its third-quarter financial results for 2024. The company is nearing completion of its FDA premarket approval application for the VenoValve, having already submitted four out of five modules. The final module is expected in the fourth quarter, with definitive data to be presented in November at the VEITHsymposium.
enVVeno also highlighted the progress of the enVVe, a non-surgical transcatheter-based valve, currently undergoing a crucial GLP study. The company aims to file for IDE approval by mid-2025 to commence a pivotal trial.
Financially, enVVeno closed the quarter with $48.4 million in cash, supported by recent fundraising efforts. The quarterly cash burn stood at $4.3 million, aligning with projections. However, net losses rose to $5.6 million, attributed to increased operational expenses.
R. E.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de EnVVeno Medical Corporation
