sur EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Updates on FDA Premarket Approval for VenoValve®
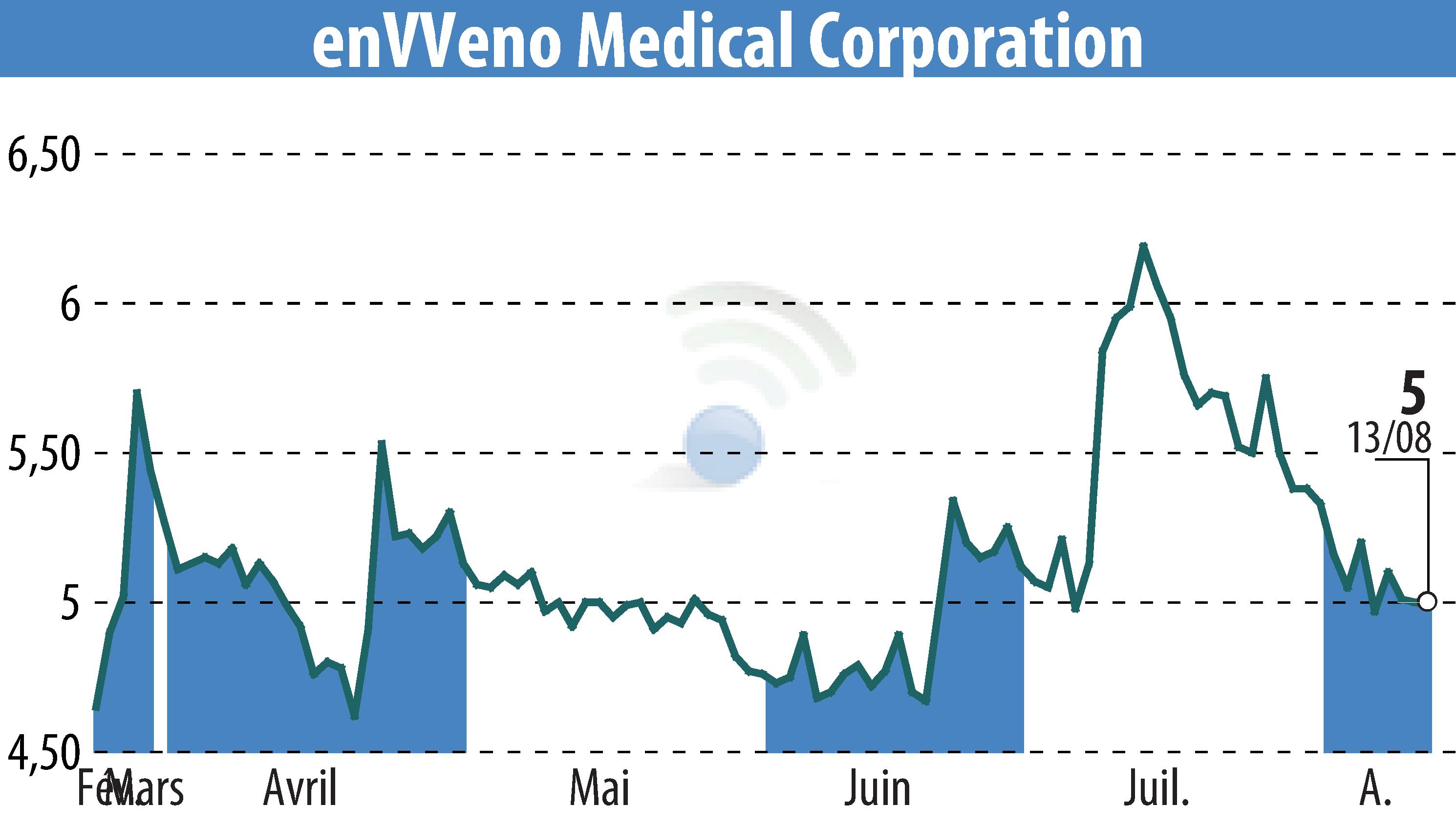
enVVeno Medical Corporation has provided an update on its FDA premarket approval (PMA) application for the VenoValve®. The application consists of five modules, and the first four have now been submitted, reviewed, and approved by the FDA. The company expects to file the fifth and final module in Q4 2024.
CEO Robert Berman expressed satisfaction with the progress, highlighting the strategic advantage of submitting modules separately to address any deficiencies early. This modular approach, implemented by the FDA in 1998, aims to enhance the efficiency of the review process.
The VenoValve is being evaluated in the SAVVE U.S. pivotal study involving 75 CVI patients across 21 U.S. sites. The final module will include clinical data from this study. enVVeno expects its existing cash reserves to support operations until the FDA's decision.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de EnVVeno Medical Corporation
