sur EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Reports Second Quarter 2024 Financial Results and Provides Corporate Update
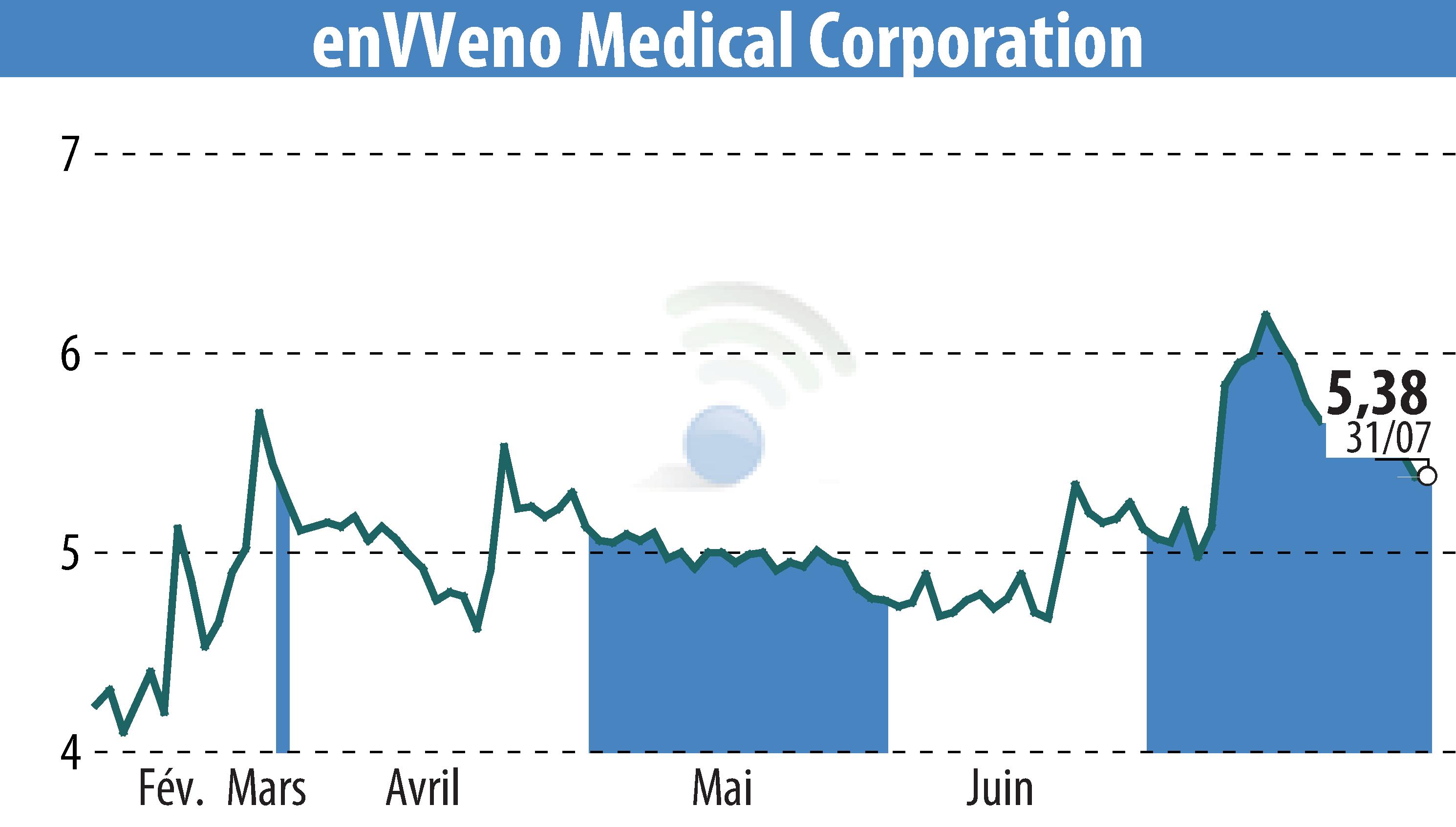
enVVeno Medical Corporation announced its financial results for Q2 2024, stating it ended the quarter with $39.1 million in cash and investments. The company expects this capital to support operations through the end of 2025, including potential approval of the VenoValve®.
Interim data demonstrated significant improvement in venous ulcer healing from the VenoValve pivotal trial. At the Society for Vascular Surgery's annual meeting, results showed 91% of patients at one year either fully healed or improved ulcers, with no recurrences.
The company plans to file for FDA approval of the VenoValve in Q4 2024 and continues progress on the enVVe® transcatheter-based valve, with a GLP study expected to commence by the end of 2024.
Financially, the company reported a net loss of $5.0 million, down from $6.5 million in Q2 2023. Research and development expenses decreased by 33.3%. Selling, general, and administrative expenses remained stable at $2.6 million.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de EnVVeno Medical Corporation
