sur EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Receives Unfavorable FDA Decision on VenoValve
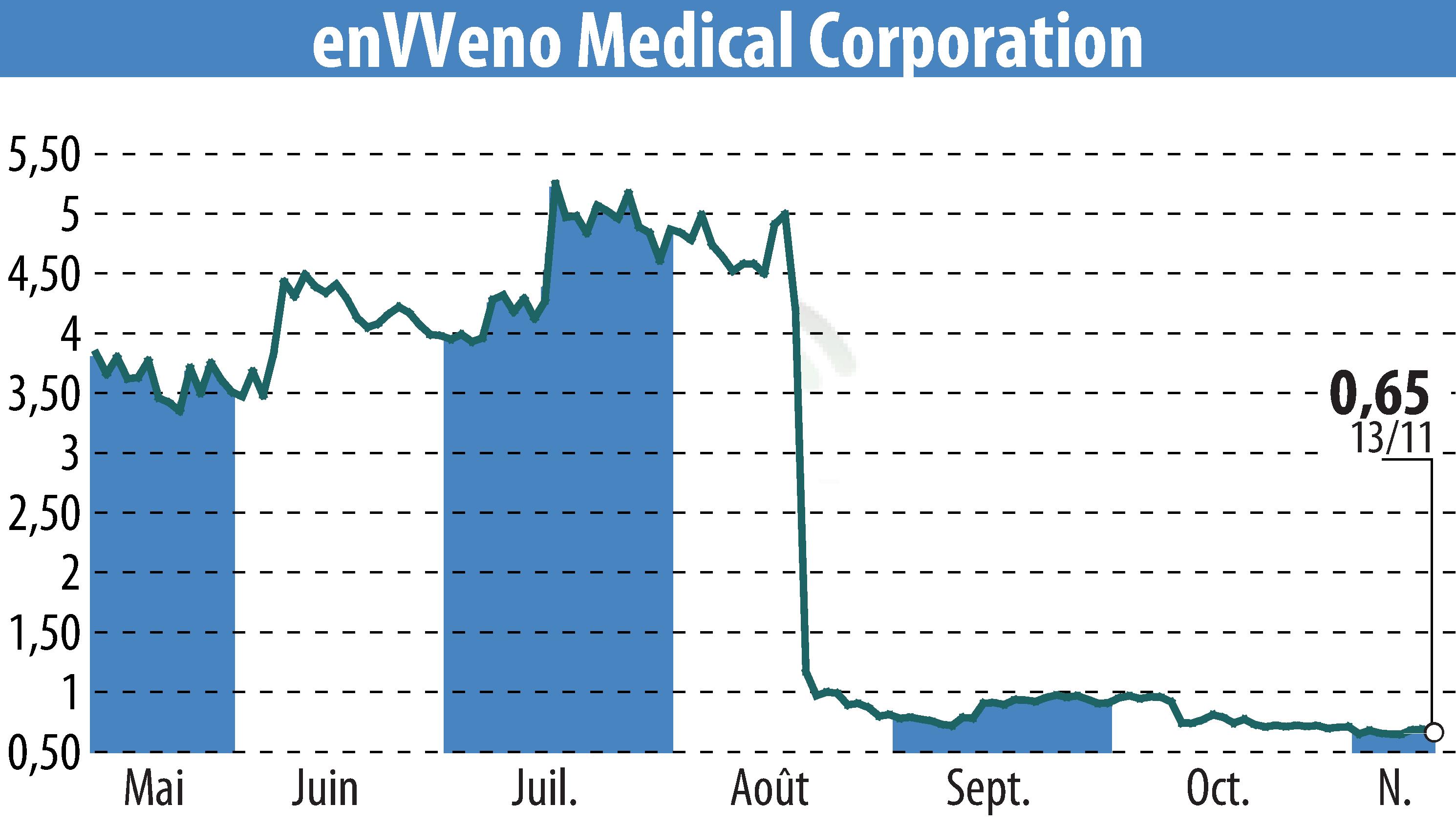
EnVVeno Medical Corporation has announced that the FDA has upheld its decision to reject the VenoValve Premarket Approval application. The surgical replacement venous valve, intended for severe deep chronic venous insufficiency (CVI), failed to meet safety and effectiveness standards. Despite the setback, CEO Robert Berman noted insights gained could guide the approval of enVVe, their next-generation device.
The company plans to shift focus to enVVe, which potentially offers a different safety profile, and is prepared for human testing. They intend to coordinate with the FDA on approval endpoints and will update as progress is made.
EnVVeno holds $31.5 million in cash and investments, sufficient to sustain operations into 2027, with quarterly expenses between $4-5 million. Chronic venous insufficiency impacts millions, with associated healthcare expenses exceeding $20 billion annually in the U.S.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de EnVVeno Medical Corporation
