sur EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Pursues FDA Supervisory Appeal
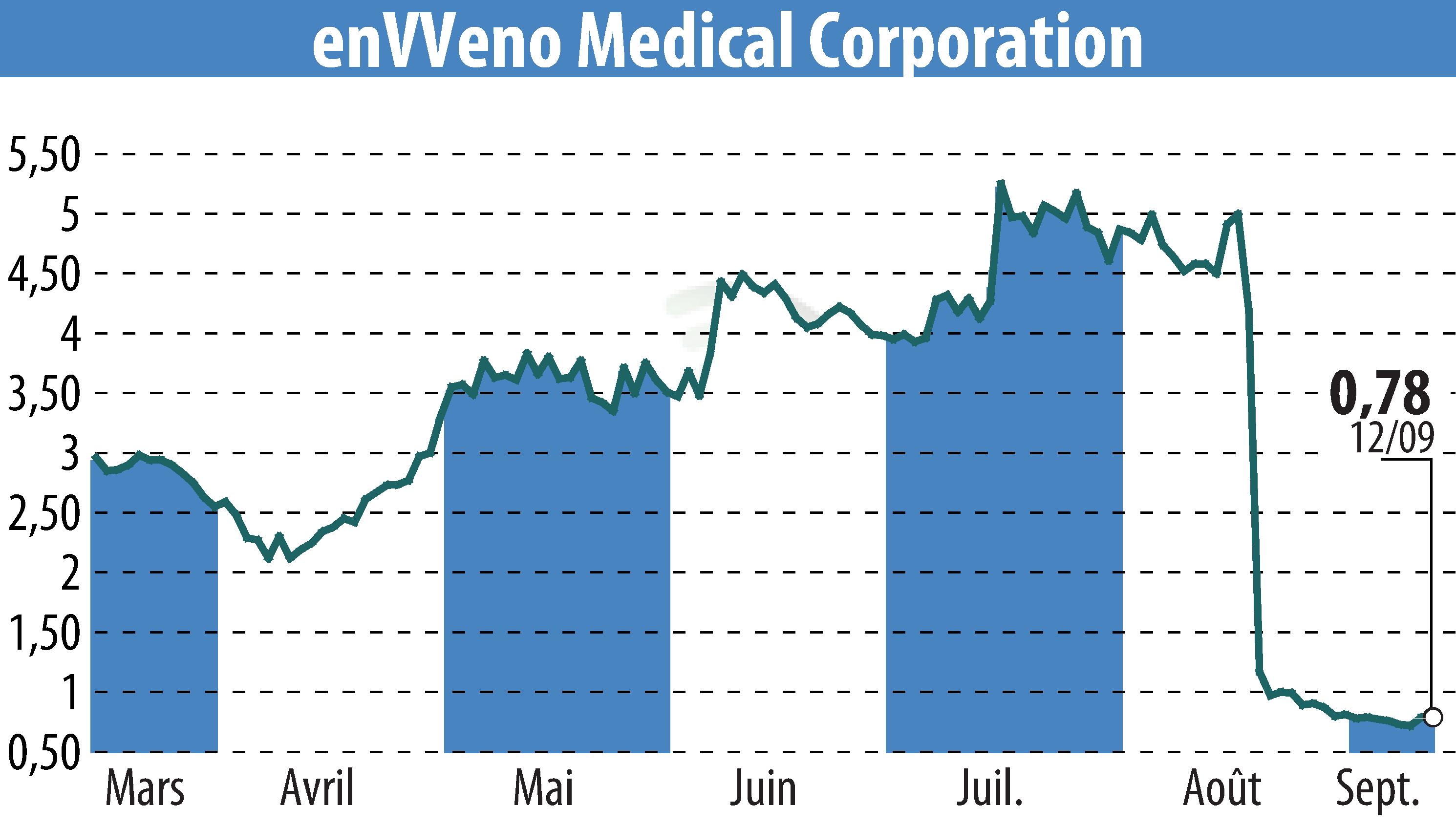
enVVeno Medical Corporation has announced plans to file a supervisory appeal concerning the FDA's initial rejection of their VenoValve's premarket approval application. The VenoValve is designed to treat severe deep chronic venous insufficiency. This decision follows a not-approvable letter from the FDA's Center for Devices and Radiological Health received on August 19, 2025.
Utilizing established mechanisms, enVVeno aims to challenge the FDA's decision within the required 30-day timeframe. The company cites its ongoing collaborative relationship with the FDA, built through previous regulatory interactions, as a foundation for optimism in seeking a positive resolution by the end of 2025.
CEO Robert Berman emphasizes the challenges inherent in navigating regulatory processes for first-in-class medical devices, reaffirming enVVeno's commitment to bringing effective treatments to U.S. patients with limited options. The appeal will focus on both physician and patient-reported data from their pivotal study.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de EnVVeno Medical Corporation
