sur EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Commences Pre-Clinical Study for enVVe Valve
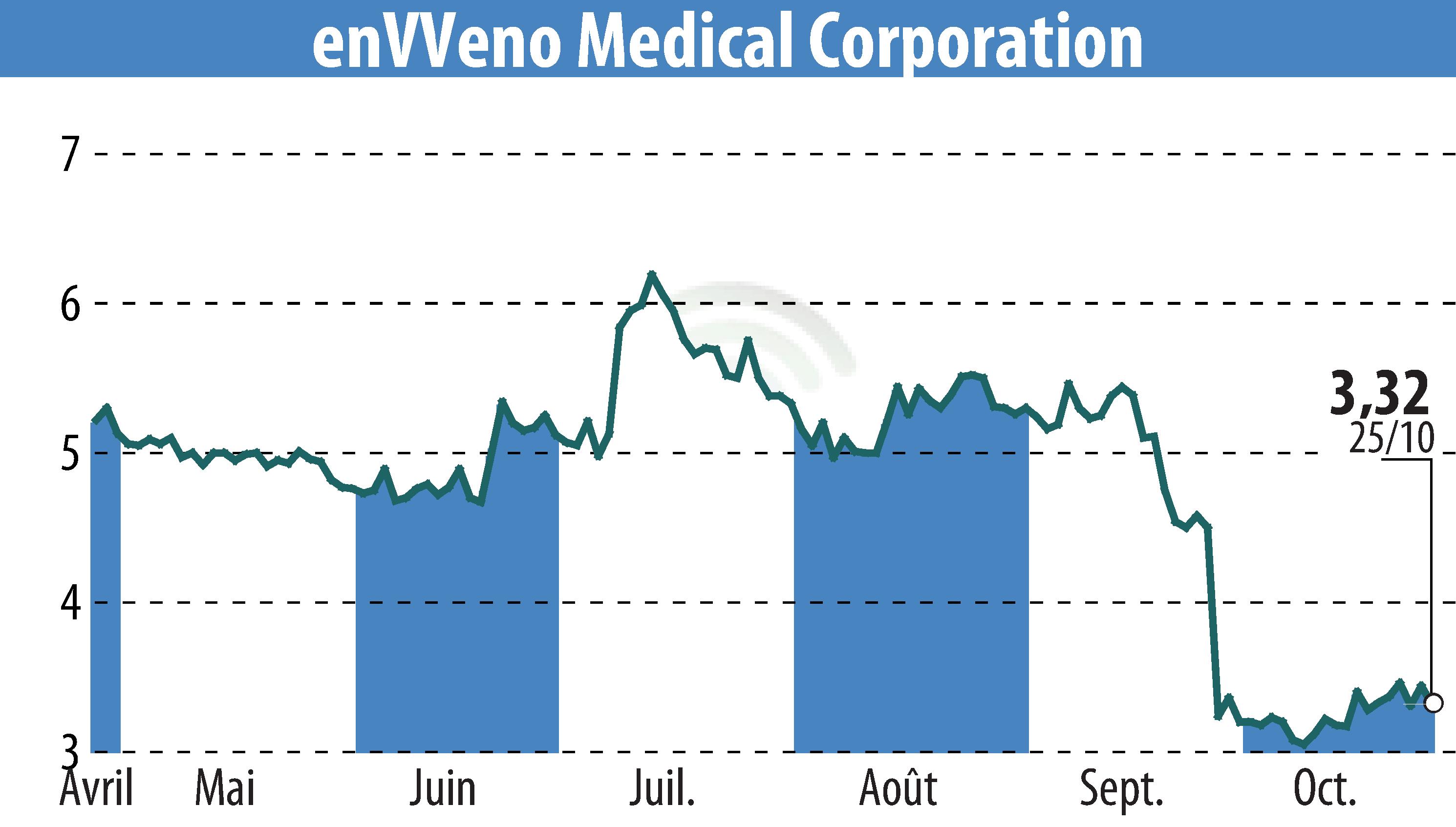
enVVeno Medical Corporation has launched a pre-clinical GLP study for its enVVe replacement venous valve. The first wave of implants using the updated delivery system has been completed successfully. This study is essential for seeking IDE approval from the FDA, with aims to apply mid-2025 for the enVVe pivotal trial.
CEO Robert Berman emphasized the technical challenges of creating a valve that fits a catheter just 0.17 inches in diameter, noting improvements in crimping and delivery systems. The upcoming GLP study results will determine the valve's efficacy.
enVVeno's main product, the VenoValve®, targets patients with severe Chronic Venous Insufficiency (CVI), a market estimated at 2.5 million candidates annually in the U.S. The enVVe valve aims for a broader reach, leveraging transcatheter technology for treatment.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de EnVVeno Medical Corporation
