sur DIAGNOSTIC MEDICAL (EPA:ALDMS)
DMS Group: FDA Clearance and U.S. Launch of !M1 System
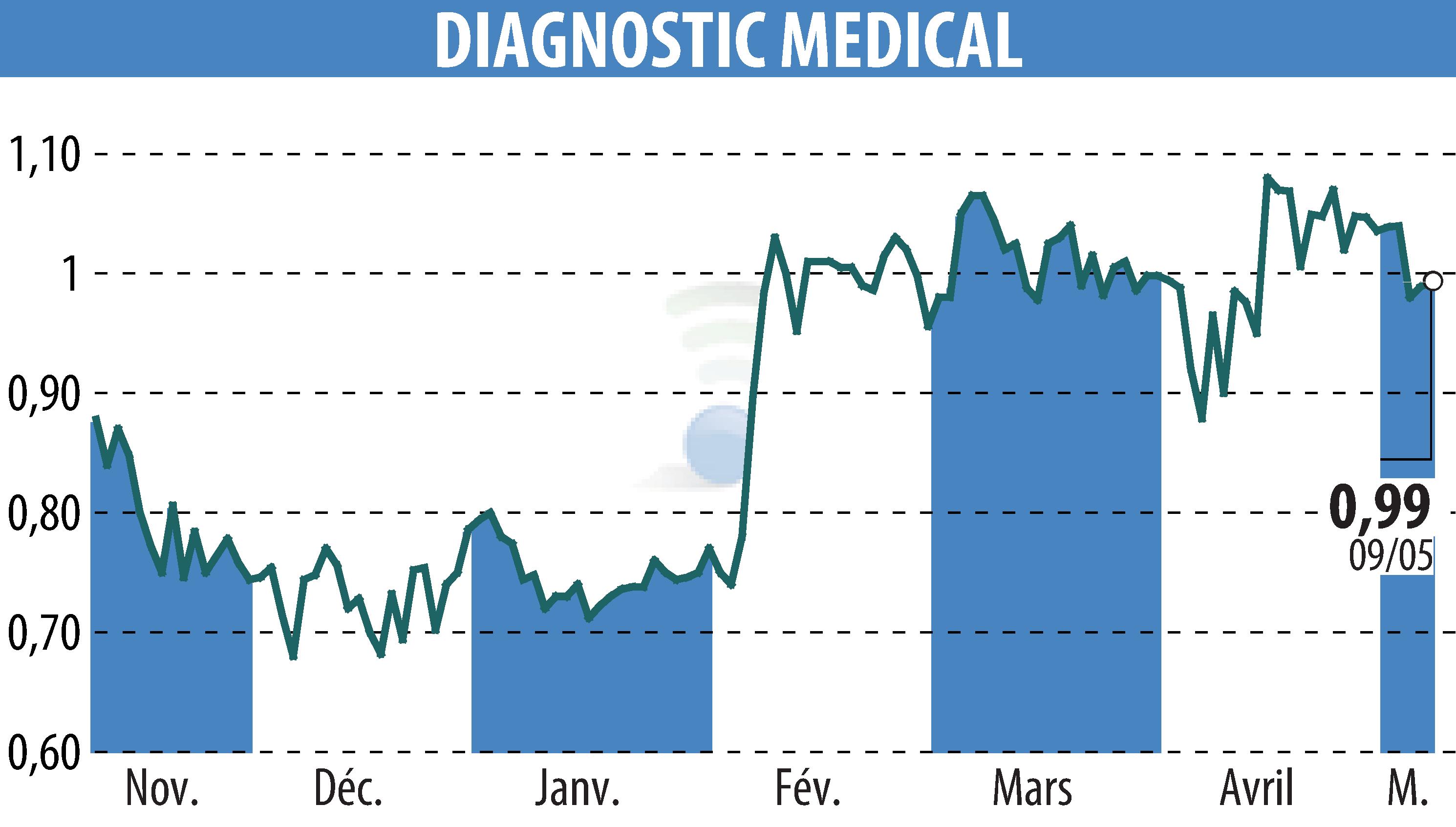
On May 12, 2025, Diagnostic Medical Systems (DMS) announced FDA clearance for their !M1 mobile radiology solution, enabling its commercialization in the U.S. market. This follows a strategic agreement with Medlink Imaging, part of Korean company Vieworks, for U.S. distribution.
Medlink Imaging will immediately begin deliveries, utilizing their nationwide network and integrating Vieworks detectors with the !M1. DMS Group CEO, Samuel Sancerni, sees this as a strategic milestone, emphasizing DMS's international growth ambitions, particularly in the dynamic U.S. mobile radiology market.
The U.S. market represents a significant opportunity, with over 1,200 mobile radiology units sold annually. The !M1, developed by Solutions for Tomorrow, is praised for its mobility, compactness, clinical performance, and adaptability to complex environments.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de DIAGNOSTIC MEDICAL
