sur CureVac (NASDAQ:CVAC)
CureVac's CVGBM Cancer Vaccine Shows Promising Results in Phase 1 Glioblastoma Study
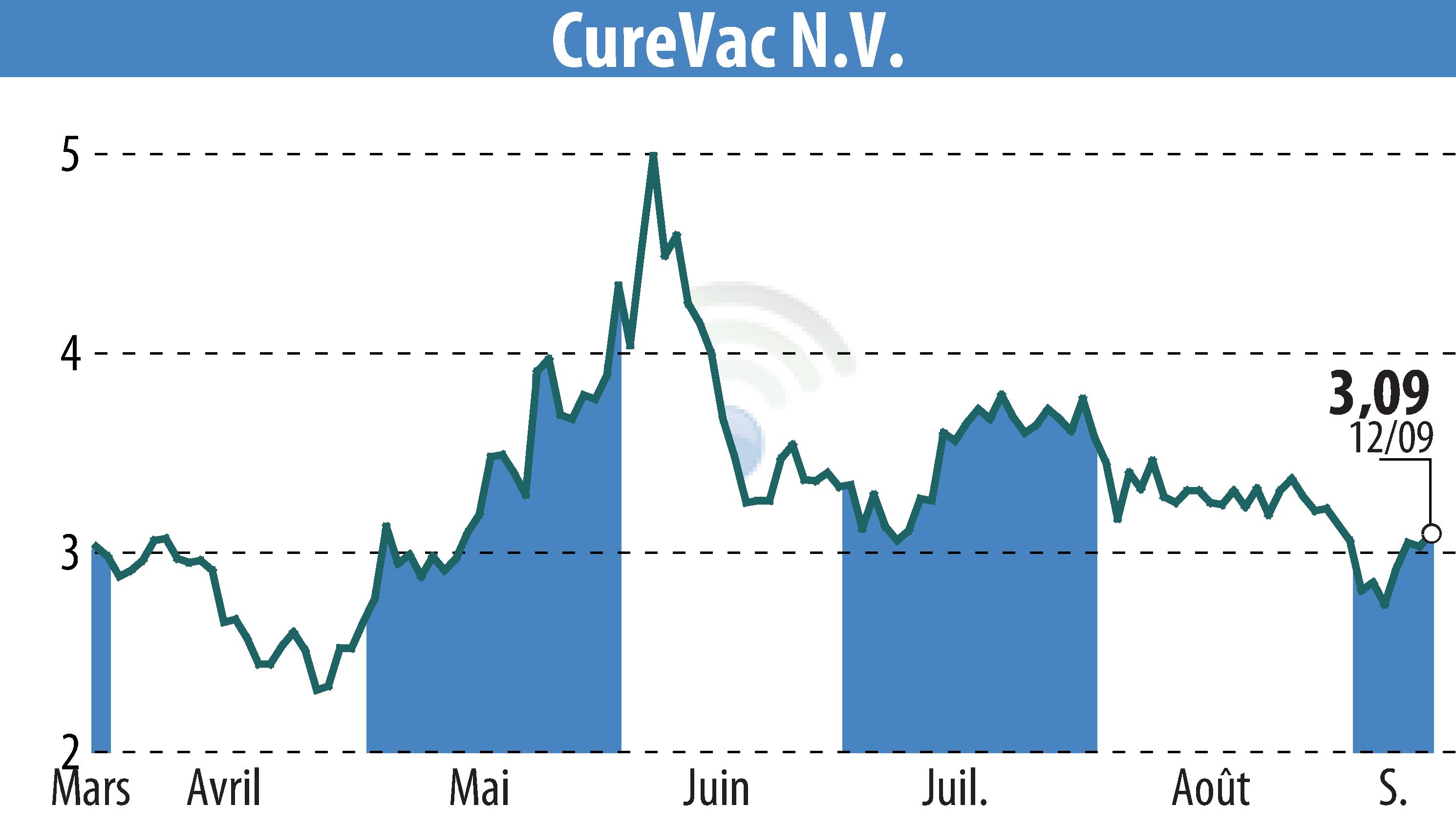
CureVac, a biopharmaceutical company, has announced encouraging preliminary results from its Phase 1 study of the CVGBM cancer vaccine presented at the ESMO 2024 Congress. The study focuses on patients with glioblastoma, an aggressive type of brain cancer.
The data demonstrated that the vaccine induced cancer antigen-specific T-cell responses in 77% of evaluable patients. Notably, 84% of these responses were de novo, meaning they were observed in patients without pre-existing T-cell activity against encoded cancer antigens. The vaccine was well tolerated, with no dose-limiting toxicities and common side effects, such as headache, fever, and chills, resolving within 1-2 days.
The CVGBM vaccine showed potential by activating both CD8+ and CD4+ T-cell responses, with the recommended dose for the upcoming dose-expansion phase set at 100 µg. CureVac anticipates further validation in the next study phase, marking a significant step in cancer immunotherapy.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de CureVac
