sur CureVac (NASDAQ:CVAC)
CureVac and GSK Report Positive Phase 2 Data for Influenza mRNA Vaccine
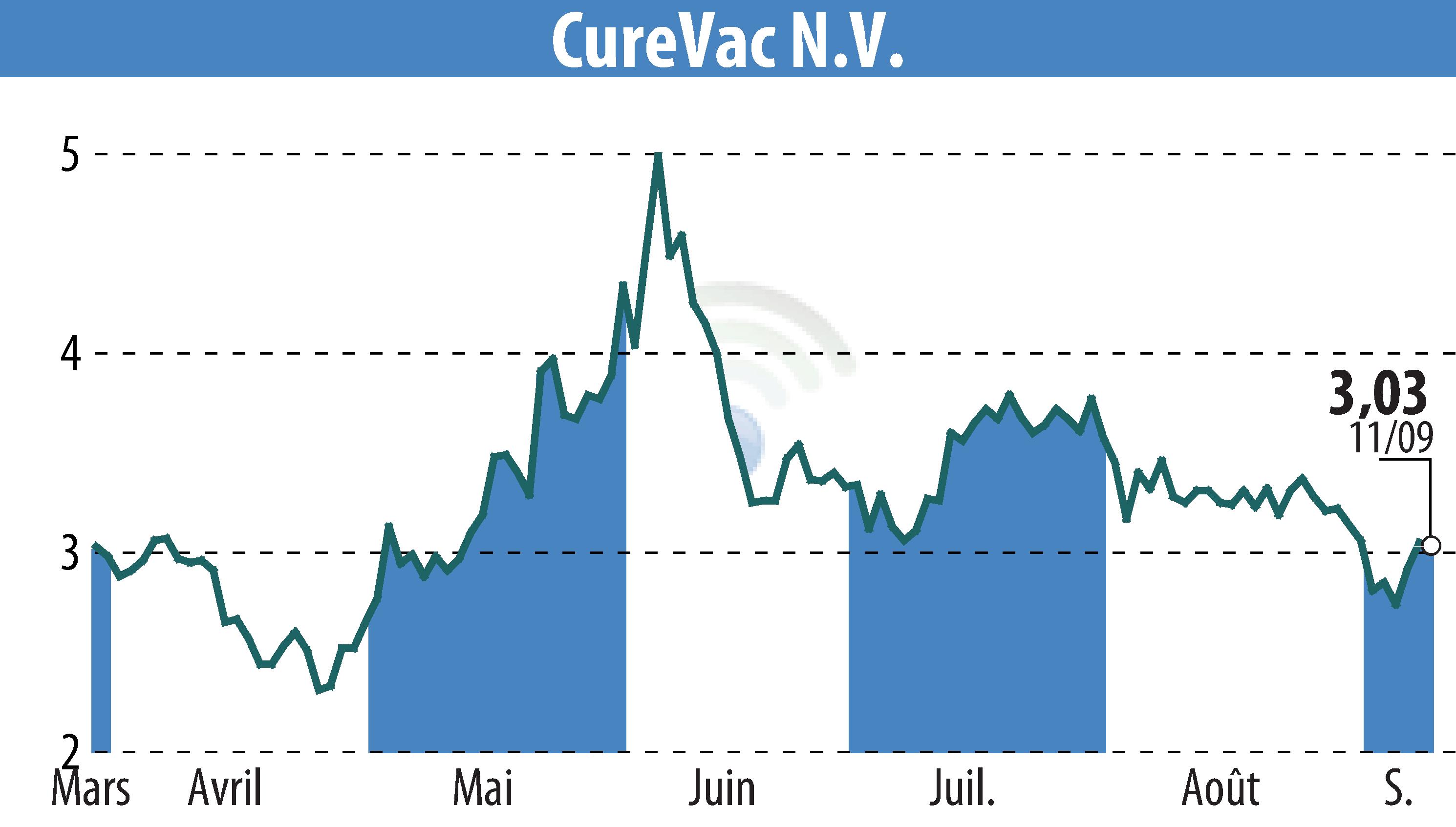
CureVac's partner GSK has announced positive Phase 2 results from their seasonal influenza mRNA vaccine program. The data showed strong immune responses to both A and B strains of influenza, meeting all predefined study endpoints. The vaccine candidate uses CureVac’s proprietary second-generation mRNA technology.
GSK's data suggests the vaccine has an acceptable safety and reactogenicity profile. This supports advancing to Phase 3, which is a significant milestone for CureVac.
In July 2024, GSK took full control of the influenza vaccine’s development, manufacturing, and commercialization under a new licensing agreement. The Phase 2 study included 500 participants, both younger and older adults, to compare immune responses.
R. E.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de CureVac
