sur CROSSJECT (EPA:ALCJ)
CROSSJECT: Progress in the EUA authorization application for ZEPIZURE®
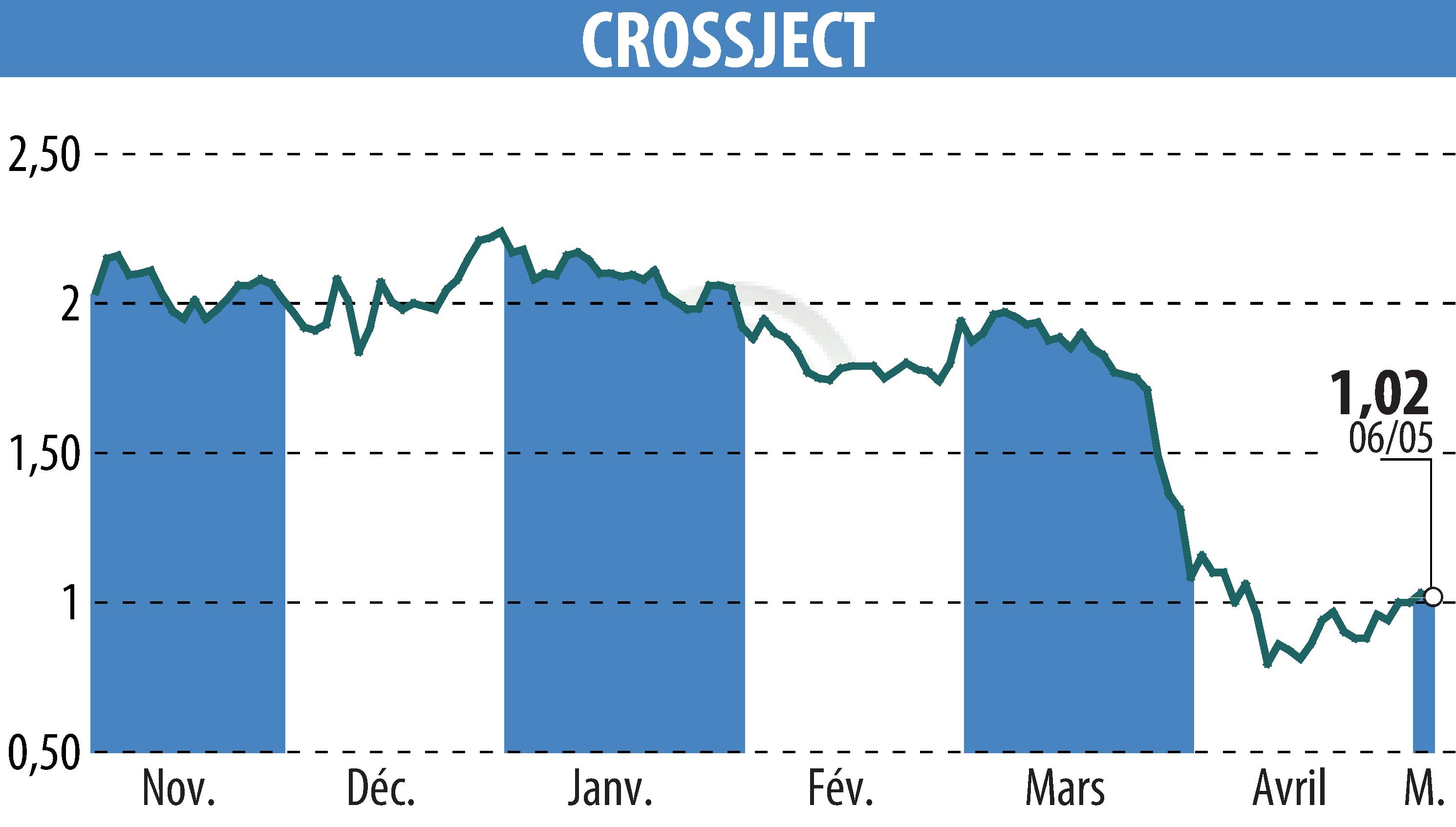
CROSSJECT announced the progress of its Emergency Use Authorization (EUA) application for its ZEPIZURE® product with the U.S. FDA. Working with EUROFINS CDMO, the company successfully completed aseptic filling of the required record batches. They plan to submit the final necessary manufacturing data by June 2025.
The company has initiated final regulatory activities to submit the ZEPIZURE® dossier under the EUA, in cooperation with BARDA, a partner in the United States. EUA batches for the CHEMPACK program for national chemical preparedness have also begun production.
CROSSJECT remains optimistic about receiving FDA clearance through its partnership with BARDA, further strengthening its ZENEO® needle-free injection technology.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de CROSSJECT
