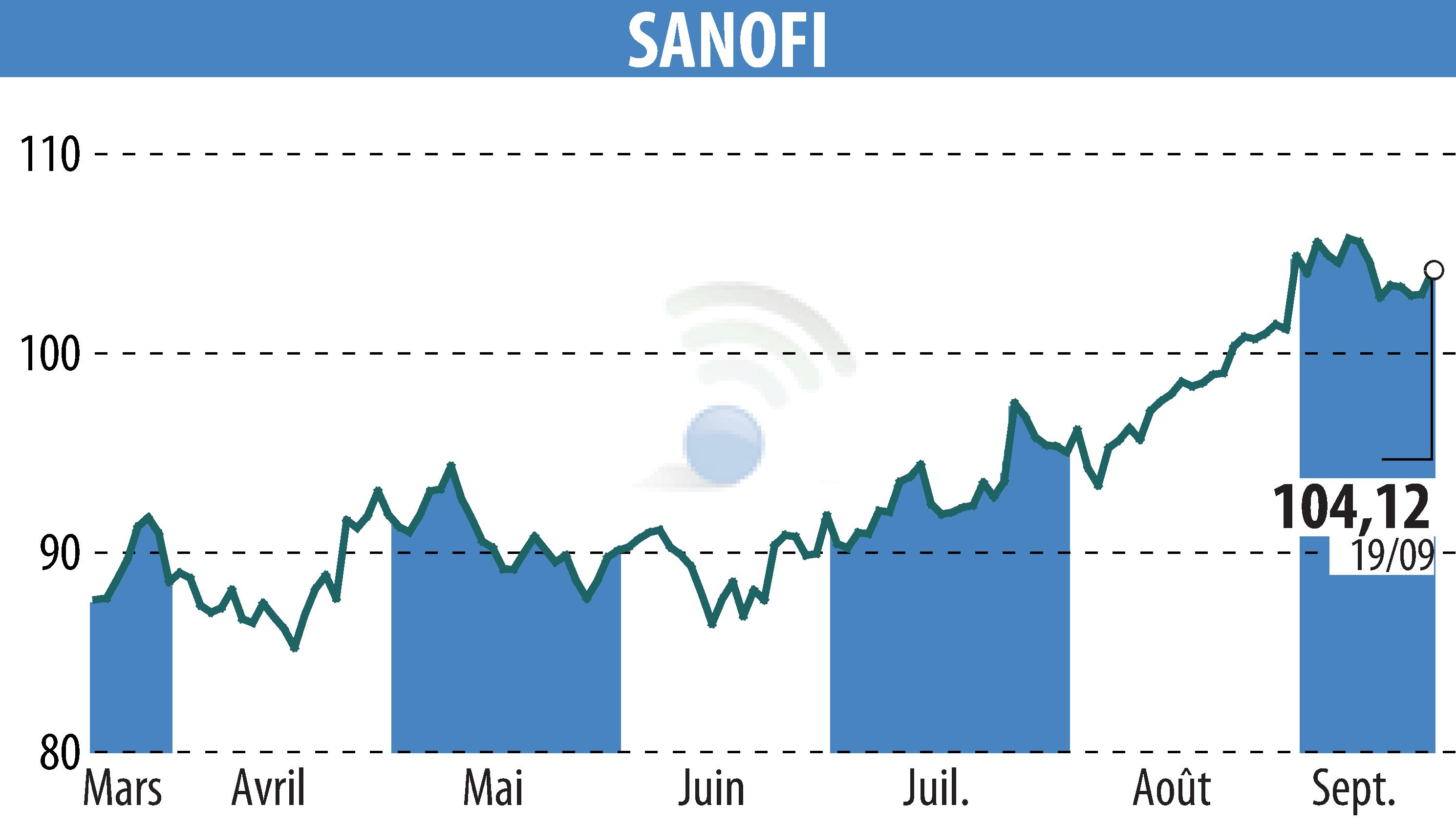
sur SANOFI-AVENTIS (EPA:SAN)
CHMP recommends approval of Dupixent for children aged 12 months and older
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency has issued a positive opinion for the approval of Dupixent (dupilumab) in the European Union. This medicine is intended for the treatment of eosinophilic oesophagitis in children aged 12 months to 11 years.
This support is based on a phase III study, showing significantly greater histological remission in children treated with Dupixent compared to placebo. Dupixent is already approved for adults and adolescents over 12 years of age.
If approved, Dupixent would become the only medicine indicated in the EU to treat this condition in this age group. The results of the study show improvements in the symptoms of oesophagitis, with a safety profile consistent with that observed in adults and adolescents.
R. E.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
