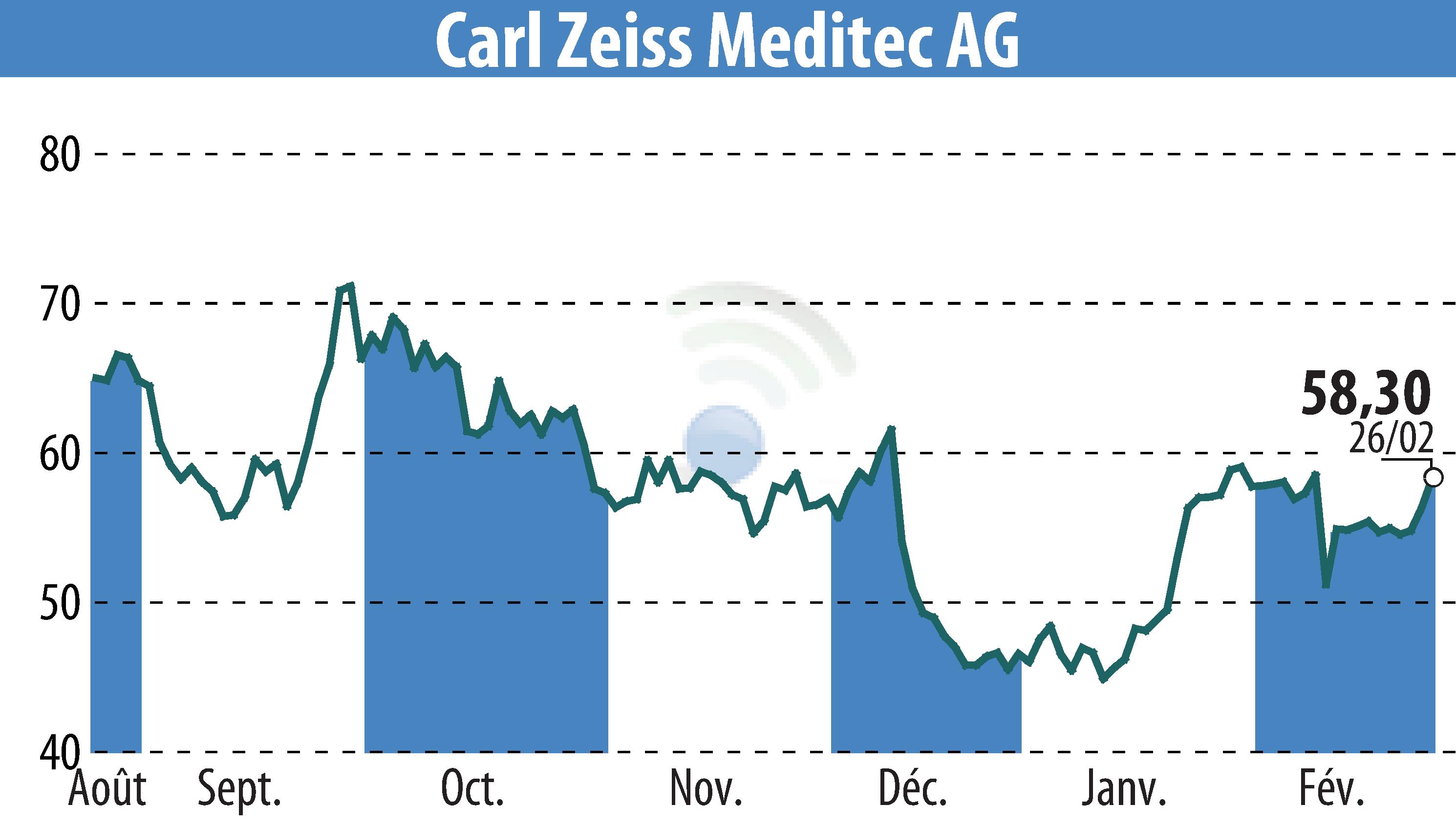
sur Carl Zeiss Meditec AG (ETR:AFX)
ZEISS VISUMAX 800 Gains China Approval
ZEISS Medical Technology has obtained approval from China's National Medical Products Administration for its VISUMAX® 800 with SMILE® pro software. This marks the entry of ZEISS's latest femtosecond laser into China's market, aiming to improve laser vision correction procedures for nearsightedness, with or without astigmatism. The technology benefits from global success, demonstrated by over 10 million surgeries performed worldwide.
The VISUMAX® 800 offers faster treatments due to a laser pulse rate of 2 MHz. This reduces procedure time, which in turn lessens stress for both patients and surgeons. Additionally, its compact design ensures compatibility with various clinical environments. Workflow is enhanced through features such as CentraLign® for easier centration and OcuLign® to counteract cyclotorsion.
ZEISS emphasizes providing advanced solutions for refractive surgeons in China, highlighted by data-driven capabilities that optimize surgical paths and efficiency. This approval represents significant market potential for laser vision correction advancements across China.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Carl Zeiss Meditec AG
