sur AMOEBA (EPA:ALMIB)
Amoéba Prepares for US Launch of AXPERA Product Line by 2025
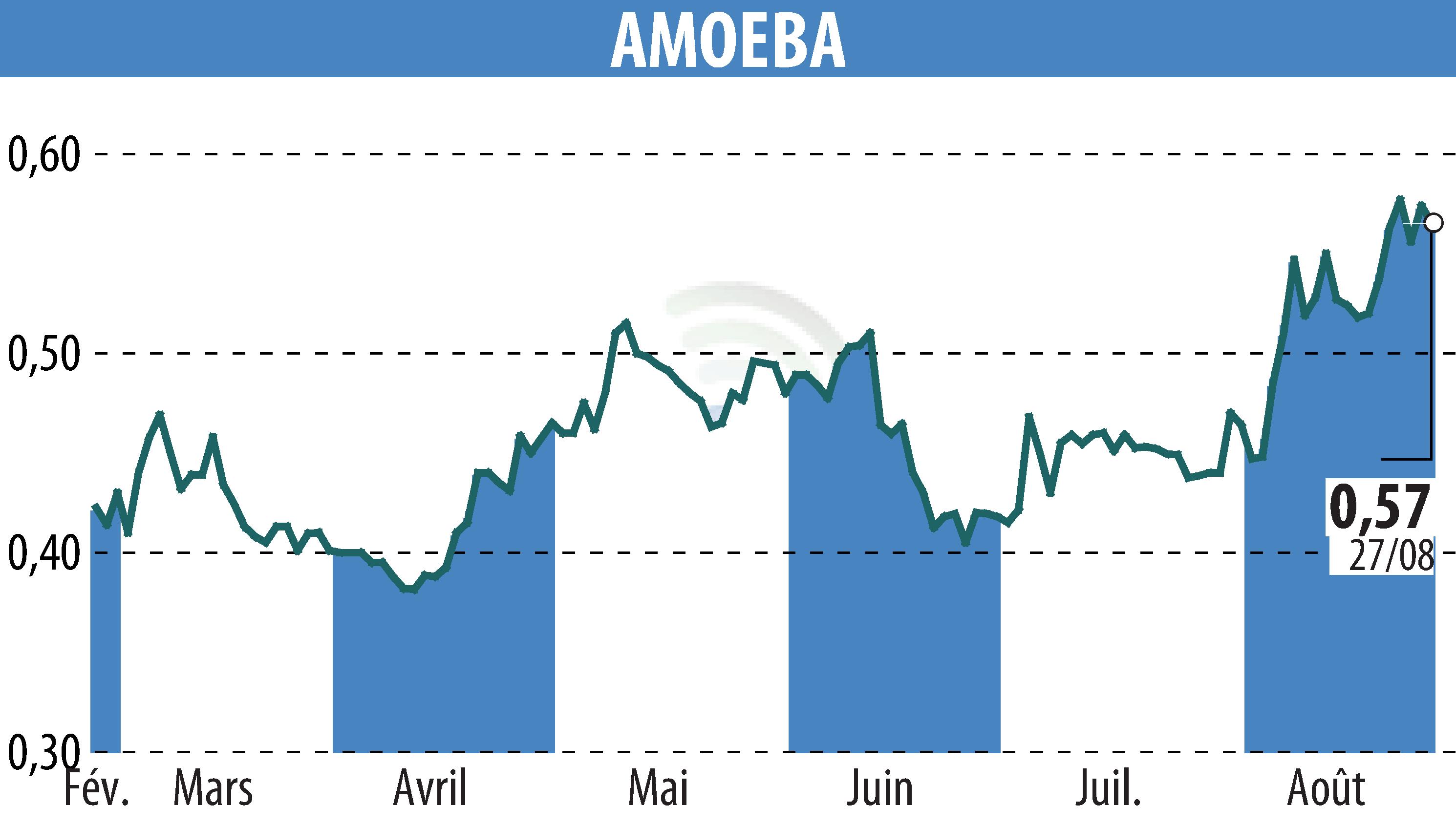
Chassieu, France, August 28, 2024 - Amoéba, a greentech firm specializing in natural microbiological solutions, announced the US Environmental Protection Agency (EPA) has deemed its biocontrol products' registration dossier complete and admissible.
Amoéba aims to accelerate its industrial and commercial development following the US approval of its biocontrol active substance. The company successfully submitted a federal market authorization application for its biofungicide products AXPERA GREEN and AXPERA NOA in July 2024. The pre-approval process has begun, with a decision anticipated by mid-2025. This would cover the entire US, except California, which has a separate registration procedure.
Alongside the regulatory process, Amoéba collaborates with Dunham Trimmer to prepare for the commercial launch of AXPERA-based solutions. Should approval be granted within the projected timeline, distribution contracts could be signed by the end of 2025.
In Europe, the European Food Safety Authority's review of the active substance dossier is nearly complete, with a final report expected by December 2024.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de AMOEBA
