sur ABIVAX (EPA:ABVX)
Abivax Reaches Key Milestone with Recruitment of 600 Patients for Phase 3 ABTECT Trial
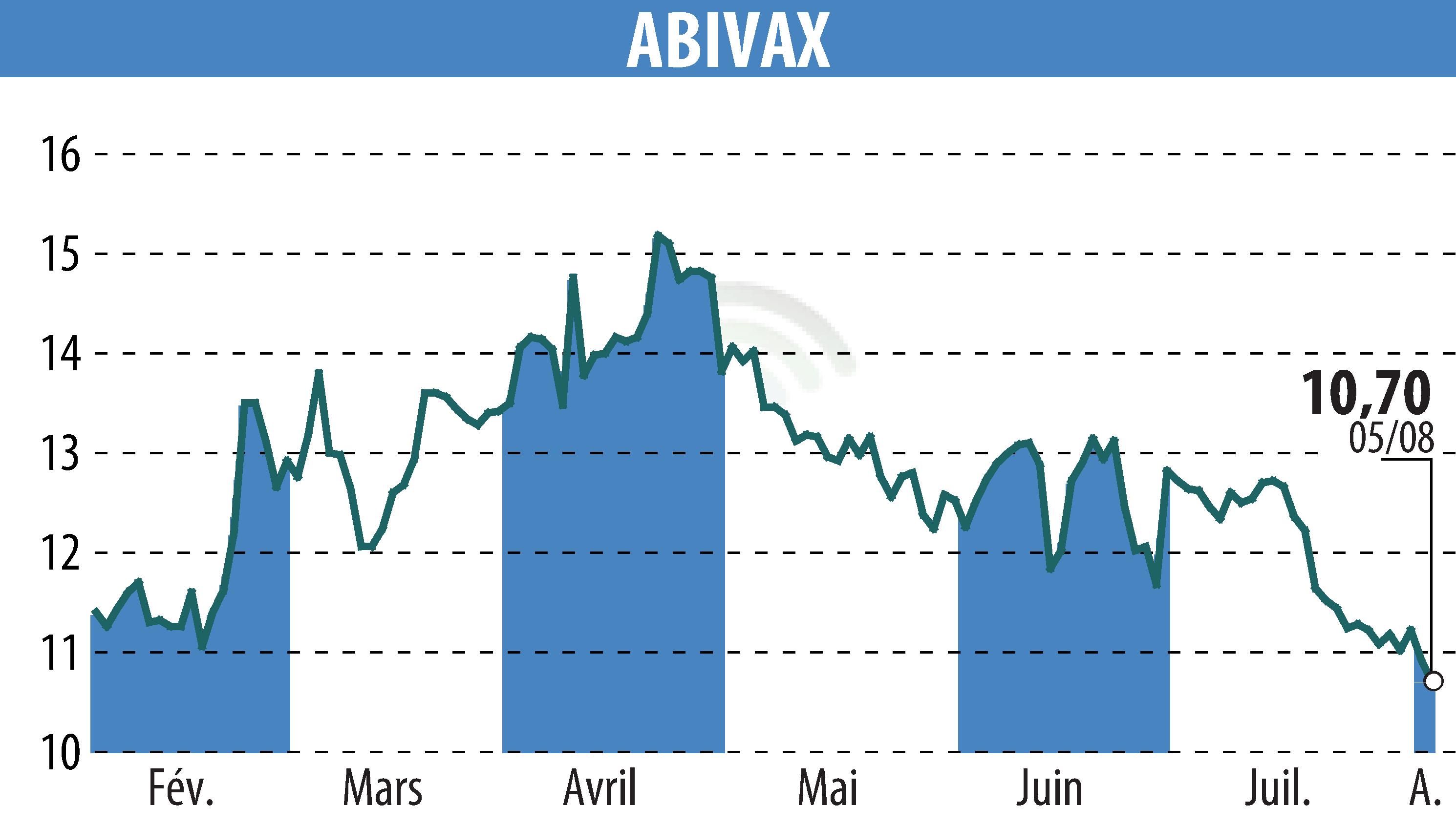
Abivax, a biotechnology company based in Paris, is continuing its phase 3 ABTECT trial of Obefazimod in the treatment of ulcerative colitis (UC). In July 2024, the company enrolled its 600th patient, approaching the end of enrollment planned for early 2025. The trial focuses on moderately to severely active UC cases.
The data collected so far shows similarities with the Phase 2b trial results, reinforcing optimism about Abivax's prospects of completing recruitment on time. The company has updated its information on its website to reflect these advancements.
Obefazimod, Abivax's lead drug candidate, has demonstrated promising efficacy in phase 2. The launch of a phase 2b clinical trial for Crohn's disease as well as the evaluation of combination therapies for the treatment of UC are also in progress. course.
R. H.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de ABIVAX
