sur ABSCIENCES (EPA:AB)
AB Science Faces Setback in EMA Approval for ALS Treatment
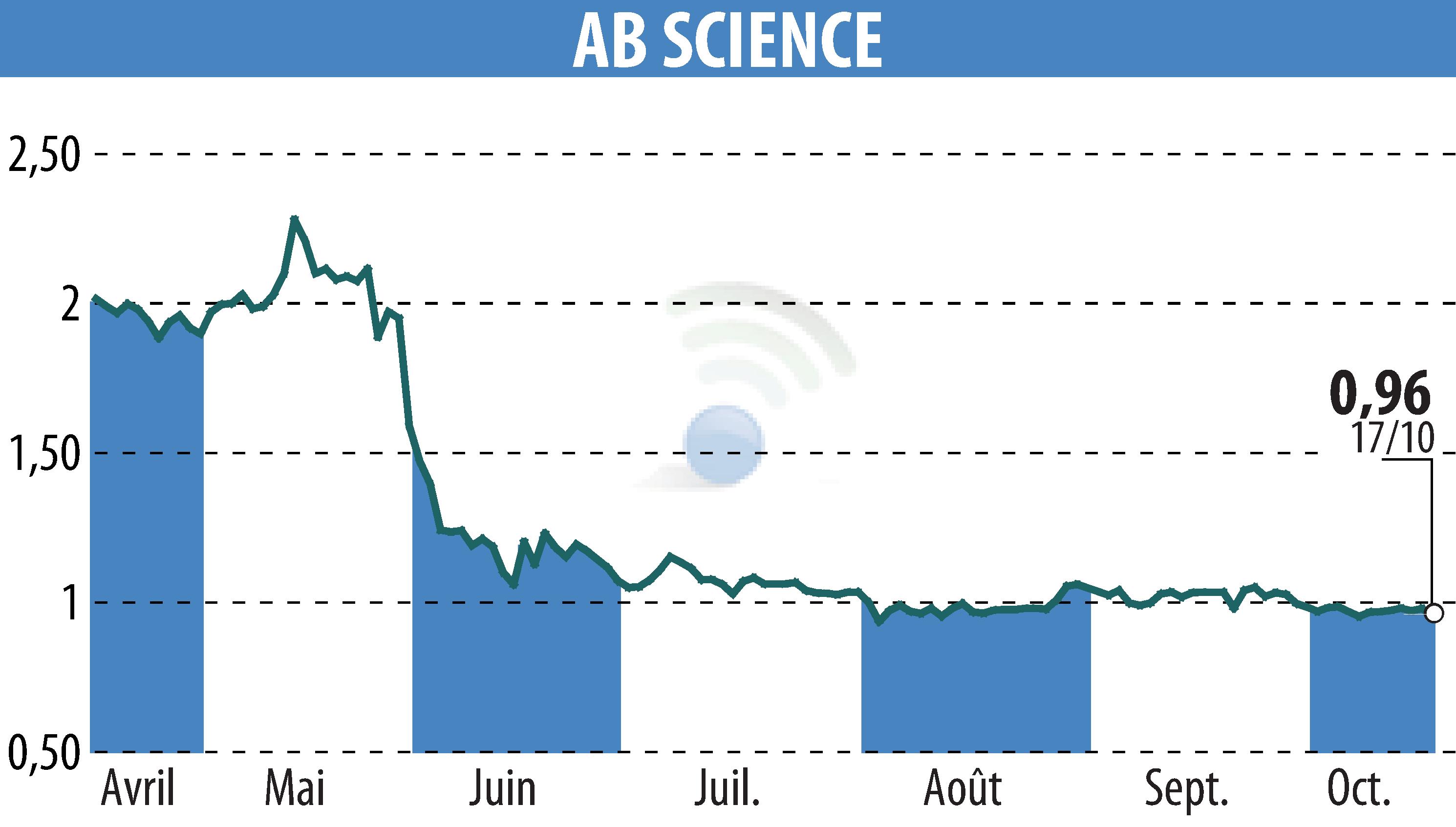
AB Science SA announced that the European Medicines Agency (EMA) has maintained its negative stance on granting conditional marketing authorization for masitinib in treating amyotrophic lateral sclerosis (ALS). The decision was made during the Committee for Medicinal Products for Human Use meeting from October 14 to 17, 2024. This follows AB Science's request for a re-examination after a similar decision in June 2024.
Despite challenges in obtaining conditional approval, AB Science pursued re-examination due to the pressing need for ALS treatment options. A previous study indicated a median survival increase of 6 months in normal progressors and a 12-month improvement in those with no complete function loss. A Scientific Advisory Group supported the study's approach but indicated that the findings alone are insufficient for authorization.
In Canada, AB Science will not pursue a reconsideration after Health Canada identified key analyses as new data. AB Science plans to concentrate on a confirmatory phase 3 program to seek full approval for masitinib in ALS.
R. P.
Copyright © 2024 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de ABSCIENCES
